Anti-inflammatory mechanisms of the novel cytokine interleukin-38 in allergic asthma
- PMID: 31645649
- PMCID: PMC7264207
- DOI: 10.1038/s41423-019-0300-7
Anti-inflammatory mechanisms of the novel cytokine interleukin-38 in allergic asthma
Abstract
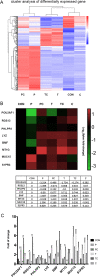
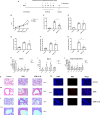
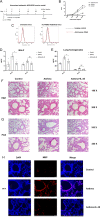
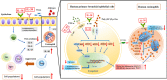
We elucidated the anti-inflammatory mechanisms of IL-38 in allergic asthma. Human bronchial epithelial cells and eosinophils were cocultured upon stimulation with the viral RLR ligand poly (I:C)/LyoVec or infection-related cytokine TNF-α to induce expression of cytokines/chemokines/adhesion molecules. House dust mite (HDM)-induced allergic asthma and humanized allergic asthma NOD/SCID murine models were established to assess anti-inflammatory mechanisms in vivo. IL-38 significantly inhibited induced proinflammatory IL-6, IL-1β, CCL5, and CXCL10 production, and antiviral interferon-β and intercellular adhesion molecule-1 expression in the coculture system. Mass cytometry and RNA-sequencing analysis revealed that IL-38 could antagonize the activation of the intracellular STAT1, STAT3, p38 MAPK, ERK1/2, and NF-κB pathways, and upregulate the expression of the host defense-related gene POU2AF1 and anti-allergic response gene RGS13. Intraperitoneal injection of IL-38 into HDM-induced allergic asthma mice could ameliorate airway hyperreactivity by decreasing the accumulation of eosinophils in the lungs and inhibiting the expression of the Th2-related cytokines IL-4, IL-5, and IL-13 in the bronchoalveolar lavage fluid (BALF) and lung homogenates. Histological examination indicated lung inflammation was alleviated by reductions in cell infiltration and goblet cell hyperplasia, together with reduced Th2, Th17, and innate lymphoid type 2 cell numbers but increased proportions of regulatory T cells in the lungs, spleen, and lymph nodes. IL-38 administration suppressed airway hyperreactivity and asthma-related IL-4 and IL-5 expression in humanized mice, together with significantly decreased CCR3+ eosinophil numbers in the BALF and lungs, and a reduced percentage of human CD4+CRTH2+ Th2 cells in the lungs and mediastinal lymph nodes. Together, our results demonstrated the anti-inflammatory mechanisms of IL-38 and provided a basis for the development of a regulatory cytokine-based treatment for allergic asthma.
Keywords: IL-38; ILC2s; Tregs; allergic asthma; eosinophils.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. - PubMed
-
- Schatz M, Rosenwasser L. The allergic asthma phenotype. J. Allergy Clin. Immunol. Pract. 2019;2:645–648. - PubMed
-
- Mckenzie GJ, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

