WZ66, a novel acetyl-CoA carboxylase inhibitor, alleviates nonalcoholic steatohepatitis (NASH) in mice
- PMID: 31645659
- PMCID: PMC7468331
- DOI: 10.1038/s41401-019-0310-0
WZ66, a novel acetyl-CoA carboxylase inhibitor, alleviates nonalcoholic steatohepatitis (NASH) in mice
Abstract
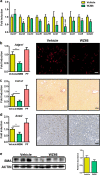
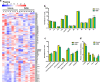
The global prevalence of nonalcoholic steatohepatitis (NASH) increases incredibly. NASH ends up to advanced liver disease, which is highly threatening to human health. Currently, treatment of NASH is very limited. Acetyl-CoA carboxylases (ACC1/ACC2) are proved as effective drug targets for NASH. We aimed to develop novel ACC inhibitors and evaluate their therapeutic value for NASH prevention. ACC inhibitors were obtained through structure-based drug design, synthesized, screened from ACC enzymatic measurement platform and elucidated in cell culture-based assays and animal models. The lipidome and microbiome analysis were integrated to assess the effects of WZ66 on lipids profiles in liver and plasma as well as gut microbiota in the intestine. WZ66 was identified as a novel ACC1/2 inhibitor. It entered systemic circulation rapidly and could accumulate in liver. WZ66 alleviated NASH-related liver features including steatosis, Kupffer cells and hepatic stellate cells activation in diet-induced obese mice. The triglycerides (TGs) and other lipids including diglycerides (DGs), phosphatidylcholine (PC) and sphingomyelin (SM) were decreased in WZ66-treated mice as evidenced by lipidome analysis in livers. The lipids profiles in plasma were also altered with WZ66 treatment. Plasma TG were moderately increased, while the activation of SREBP1c was not detected. WZ66 also downregulated the abundance of Allobaculum, Mucispirillum and Prevotella genera as well as Mucispirillum schaedleri species in gut microbiota. WZ66 is an ideal lead compound and a potential drug candidate deserving further investigation in the therapeutics of NASH.
Keywords: Acetyl-CoA carboxylase; WZ66; gut microbiome; nonalcoholic steatohepatitis; pharmacokinetics lipidome.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

