Wide-field time-correlated single photon counting-based fluorescence lifetime imaging microscopy
- PMID: 31645797
- PMCID: PMC6716551
- DOI: 10.1016/j.nima.2019.162365
Wide-field time-correlated single photon counting-based fluorescence lifetime imaging microscopy
Abstract
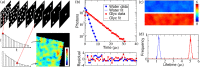
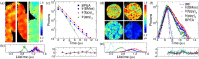
Wide-field time-correlated single photon counting detection techniques, where the position and the arrival time of the photons are recorded simultaneously using a camera, have made some advances recently. The technology and instrumentation used for this approach is employed in areas such as nuclear science, mass spectroscopy and positron emission tomography, but here, we discuss some of the wide-field TCSPC methods, for applications in fluorescence microscopy. We describe work by us and others as presented in the Ulitima fast imaging and tracking conference at the Argonne National Laboratory in September 2018, from phosphorescence lifetime imaging (PLIM) microscopy on the microsecond time scale to fluorescence lifetime imaging (FLIM) on the nanosecond time scale, and highlight some applications of these techniques.
© 2019 The Authors.
Figures


References
-
- Suhling K., Hirvonen L.M., Levitt J.A., Chung P.-H., Tregidgo C., Le Marois A., Rusakov D.A., Zheng K., Ameer-Beg S., Poland S., Coelho S., Henderson R., Krstajic N. Fluorescence lifetime imaging (FLIM): Basic concepts and some recent developments. Med. Photonics. 2015;27:3–40.
-
- Shcheslavskiy V.I., Neubauer A., Bukowiecki R., Dinter F., Becker W. Combined fluorescence and phosphorescence lifetime imaging. Appl. Phys. Lett. 2016;108(9)
-
- Baggaley E., Botchway S.W., Haycock J.W., Morris H., Sazanovich I.V., Williams J.A.G., Weinstein J.A. Long-lived metal complexes open up microsecond lifetime imaging microscopy under multiphoton excitation: from FLIM to PLIM and beyond. Chem. Sci. 2014;5(3):879–886.
-
- O’Connor D.V., Phillips D. Academic Press; London: 1984. Time-Correlated Single-Photon Counting.
-
- Bothe W., Kolhörster W. Das Wesen der Höhenstrahlung. Z. Phys. 1929;56(11):751–777.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
