CD80 expression promotes immune surveillance in Barrett's metaplasia
- PMID: 31646078
- PMCID: PMC6791427
- DOI: 10.1080/2162402X.2019.1636618
CD80 expression promotes immune surveillance in Barrett's metaplasia
Abstract
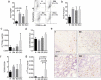
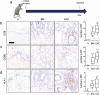
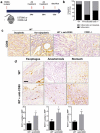
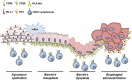
Esophageal adenocarcinoma (EAC) is the final step of a pathway starting with esophageal reflux disease, Barrett's metaplasia and Barrett's dysplasia. Positive costimulatory ligands such as CD80 have been suggested to contribute to anti-tumor T-cell efficacy. Here we report for the first time the role of CD80 in the inflammatory esophageal carcinogenesis and characterize the immune environment of EAC. Mucosa samples from cancer were obtained during esophagectomy from patients affected by EAC. Fresh biopsies were obtained from patients who underwent endoscopy for screening or follow-up. A rodent model of reflux induced esophageal carcinogenesis was created with an esophago-gastro-jejunostomy. CD80 expression was increased in epithelial cells during metaplasia in the inflammatory esophageal carcinogenesis cascade. Cd80 null mice as well as WT mice that received antiCD80 antibodies showed a higher rate of dysplasia and KI-67+ cells. These results suggest that CD80 mediates an active immune surveillance process in early inflammation-driven esophageal carcinogenesis.
Keywords: Barrett’s esophagus; CD80; adenocarcinoma; esophageal dysplasia; immune surveillance.
© 2019 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures
References
-
- Noffsinger A. Neoplastic risk assessment in Barrett’s esophagus: how far have come? Hum Pathol. 2003;34:965–967. - PubMed
-
- Schnell TG, Sontag SJ, Chejfec G, Aranha G, Metz A, O’Connell S, Seidel UJ, Sonnenberg A. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. - PubMed
-
- Conio M, Blanchi S, Lapertosa G, Ferraris R, Sablich R, Marchi S, D’Onofrio V, Lacchin T, Iaquinto G, Missale G, et al Long-term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–1939. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
