Trial watch: dendritic cell vaccination for cancer immunotherapy
- PMID: 31646087
- PMCID: PMC6791419
- DOI: 10.1080/2162402X.2019.1638212
Trial watch: dendritic cell vaccination for cancer immunotherapy
Abstract
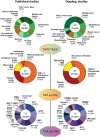
Dendritic- cells (DCs) have received considerable attention as potential targets for the development of anticancer vaccines. DC-based anticancer vaccination relies on patient-derived DCs pulsed with a source of tumor-associated antigens (TAAs) in the context of standardized maturation-cocktails, followed by their reinfusion. Extensive evidence has confirmed that DC-based vaccines can generate TAA-specific, cytotoxic T cells. Nonetheless, clinical efficacy of DC-based vaccines remains suboptimal, reflecting the widespread immunosuppression within tumors. Thus, clinical interest is being refocused on DC-based vaccines as combinatorial partners for T cell-targeting immunotherapies. Here, we summarize the most recent preclinical/clinical development of anticancer DC vaccination and discuss future perspectives for DC-based vaccines in immuno-oncology.
Keywords: Antigen cross-presentation; DAMPs; TLR signaling; clinical trial; immune checkpoint blockers; plasmacytoid dendritic cells; tumor-infiltrating lymphocytes.
© 2019 Taylor & Francis Group, LLC.
Figures
Similar articles
-
Trial watch: Dendritic cell (DC)-based immunotherapy for cancer.Oncoimmunology. 2022 Jul 4;11(1):2096363. doi: 10.1080/2162402X.2022.2096363. eCollection 2022. Oncoimmunology. 2022. PMID: 35800158 Free PMC article.
-
Trial watch: Dendritic cell-based anticancer immunotherapy.Oncoimmunology. 2017 May 12;6(7):e1328341. doi: 10.1080/2162402X.2017.1328341. eCollection 2017. Oncoimmunology. 2017. PMID: 28811970 Free PMC article. Review.
-
Trial watch: anticancer vaccination with dendritic cells.Oncoimmunology. 2024 Oct 9;13(1):2412876. doi: 10.1080/2162402X.2024.2412876. eCollection 2024. Oncoimmunology. 2024. PMID: 39398476 Free PMC article. Review.
-
Trial watch: Dendritic cell-based anticancer therapy.Oncoimmunology. 2014 Dec 21;3(11):e963424. doi: 10.4161/21624011.2014.963424. eCollection 2014 Nov. Oncoimmunology. 2014. PMID: 25941593 Free PMC article. Review.
-
Eliciting cytotoxic T lymphocytes against human laryngeal cancer-derived antigens: evaluation of dendritic cells pulsed with a heat-treated tumor lysate and other antigen-loading strategies for dendritic-cell-based vaccination.J Exp Clin Cancer Res. 2016 Jan 22;35:18. doi: 10.1186/s13046-016-0295-1. J Exp Clin Cancer Res. 2016. PMID: 26795730 Free PMC article.
Cited by
-
Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors.Nat Rev Clin Oncol. 2020 Dec;17(12):725-741. doi: 10.1038/s41571-020-0413-z. Epub 2020 Aug 5. Nat Rev Clin Oncol. 2020. PMID: 32760014 Review.
-
Integration of the PD-L1 inhibitor atezolizumab and WT1/DC vaccination into standard-of-care first-line treatment for patients with epithelioid malignant pleural mesothelioma-Protocol of the Immuno-MESODEC study.PLoS One. 2024 Jul 15;19(7):e0307204. doi: 10.1371/journal.pone.0307204. eCollection 2024. PLoS One. 2024. PMID: 39008481 Free PMC article.
-
New Approaches to Dendritic Cell-Based Therapeutic Vaccines Against HIV-1 Infection.Front Immunol. 2022 Jan 4;12:719664. doi: 10.3389/fimmu.2021.719664. eCollection 2021. Front Immunol. 2022. PMID: 35058917 Free PMC article. Review.
-
Emerging biomaterial-based strategies for personalized therapeutic in situ cancer vaccines.Biomaterials. 2022 Jan;280:121297. doi: 10.1016/j.biomaterials.2021.121297. Epub 2021 Nov 30. Biomaterials. 2022. PMID: 34902729 Free PMC article. Review.
-
Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy.J Exp Clin Cancer Res. 2020 May 18;39(1):89. doi: 10.1186/s13046-020-01586-y. J Exp Clin Cancer Res. 2020. PMID: 32423420 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
