Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients
- PMID: 31646105
- PMCID: PMC6791460
- DOI: 10.1080/2162402X.2019.1655964
Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients
Abstract
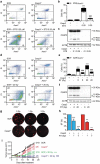
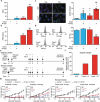
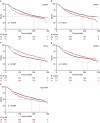
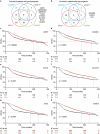
Caspase 3 (CASP3) has a key role in the execution of apoptosis, and many cancer cells are believed to disable CASP3 as a mechanism of resistance to cytotoxic therapeutics. Alongside, CASP3 regulates stress-responsive immunomodulatory pathways, including secretion of type I interferon (IFN). Here, we report that mouse mammary carcinoma TSA cells lacking Casp3 or subjected to chemical caspase inhibition were as sensitive to the cytostatic and cytotoxic effects of radiation therapy (RT) in vitro as their control counterparts, yet secreted increased levels of type I IFN. This effect originated from the accrued accumulation of irradiated cells with cytosolic DNA, likely reflecting the delayed breakdown of cells experiencing mitochondrial permeabilization in the absence of CASP3. Casp3-/- TSA cells growing in immunocompetent syngeneic mice were more sensitive to RT than their CASP3-proficient counterparts, and superior at generating bona fide abscopal responses in the presence of an immune checkpoint blocker. Finally, multiple genetic signatures of apoptotic proficiency were unexpectedly found to have robust negative (rather than positive) prognostic significance in a public cohort of breast cancer patients. However, these latter findings were not consistent with genetic signatures of defective type I IFN signaling, which were rather associated with improved prognosis. Differential gene expression analysis on patient subgroups with divergent prognosis (as stratified by independent signatures of apoptotic proficiency) identified SLC7A2 as a new biomarker with independent prognostic value in breast cancer patients. With the caveats associated with the retrospective investigation of heterogeneous, public databases, our data suggest that apoptotic caspases may influence the survival of breast cancer patients (or at least some subsets thereof) via mechanisms not necessarily related to type I IFN signaling as they identify a novel independent prognostic biomarker that awaits prospective validation.
Keywords: AGR3; APAF1; BCL2; CASP9; STC2; STING; SUSD3.
© 2019 Taylor & Francis Group, LLC.
Figures


Comment in
-
Targeting Apoptosis Inhibition to Activate Antitumor Immunity.Trends Immunol. 2019 Dec;40(12):1073-1075. doi: 10.1016/j.it.2019.11.002. Epub 2019 Nov 14. Trends Immunol. 2019. PMID: 31735511 Free PMC article.
-
Premortem Tumor Stress in Radioimmunotherapy.Trends Cancer. 2020 Mar;6(3):173-174. doi: 10.1016/j.trecan.2020.01.001. Epub 2020 Jan 27. Trends Cancer. 2020. PMID: 32101719
References
-
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi:10.1038/s41418-017-0012-4. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
