A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice
- PMID: 31646107
- PMCID: PMC6791453
- DOI: 10.1080/2162402X.2019.1657375
A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice
Abstract
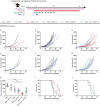
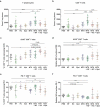
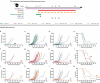
We have recently shown that chemotherapy with immunogenic cell death (ICD)-inducing agents can be advantageously combined with fasting regimens or caloric restriction mimetics (CRMs) to achieve superior tumor growth control via a T cell-dependent mechanism. Here, we show that the blockade of the CD11b-dependent extravasation of myeloid cells blocks such a combination effect as well. Based on the characterization of the myeloid and lymphoid immune infiltrates, including the expression pattern of immune checkpoint proteins (and noting a chemotherapy-induced overexpression of programmed death-ligand 1, PD-L1, on both cancer cells and leukocytes, as well as a reduced frequency of exhausted CD8+ T cells positive for programmed cell death 1 protein, PD-1), we then evaluated the possibility to combine ICD inducers, CRMs and targeting of the PD-1/PD-L1 interaction. While fasting or CRMs failed to improve tumor growth control by PD-1 blockade, ICD inducers alone achieved a partial sensitization to treatment with a PD-1-specific antibody. However, definitive cure of most of the tumor-bearing mice was only achieved by a tritherapy combining (i) ICD inducers exemplified by mitoxantrone and oxaliplatin, (ii) CRMs exemplified by hydroxycitrate and spermidine and substitutable for by fasting, and (iii) immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 interaction. Altogether, these results point to the possibility of synergistic interactions among distinct classes of anticancer agents.
Keywords: Caloric restriction mimetics; chemotherapy; combination therapies; immune checkpoint blockers; tumor immune infiltrate.
© 2019 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures




References
-
- Wallis CJD, Butaney M, Satkunasivam R, Freedland SJ, Patel SP, Hamid O, Pal SK, Klaassen Z.. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 2019. doi:10.1001/jamaoncol.2018.5904. - DOI - PMC - PubMed
-
- Wei SC, Duffy CR, Allison JP.. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1–15. doi:10.1158/2159-8290.CD-18-0367. - DOI - PubMed
-
- Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G, Szustakowski JD, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33:853–861.e4. doi:10.1016/j.ccell.2018.04.001. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
