Evolutionary and functional insights into the mechanism underlying body-size-related adaptation of mammalian hemoglobin
- PMID: 31647054
- PMCID: PMC6812962
- DOI: 10.7554/eLife.47640
Evolutionary and functional insights into the mechanism underlying body-size-related adaptation of mammalian hemoglobin
Abstract
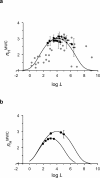
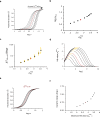
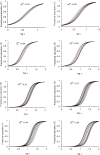
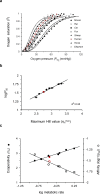
Hemoglobin (Hb) represents a model protein to study molecular adaptation in vertebrates. Although both affinity and cooperativity of oxygen binding to Hb affect tissue oxygen delivery, only the former was thought to determine molecular adaptations of Hb. Here, we suggest that Hb affinity and cooperativity reflect evolutionary and physiological adaptions that optimized tissue oxygen delivery. To test this hypothesis, we derived the relationship between the Hill coefficient and the relative affinity and conformational changes parameters of the Monod-Wymann-Changeux allosteric model and graphed the 'biophysical Hill landscape' describing this relation. We found that mammalian Hb cooperativity values all reside on a ridge of maximum cooperativity along this landscape that allows for both gross- and fine-tuning of tissue oxygen unloading to meet the distinct metabolic requirements of mammalian tissues for oxygen. Our findings reveal the mechanism underlying body size-related adaptation of mammalian Hb. The generality and implications of our findings are discussed.
Keywords: MWC; adaptation; allostery; biochemistry; chemical biology; cooperativity; evolution; mechanism; none.
© 2019, Rapp and Yifrach.
Conflict of interest statement
OR, OY No competing interests declared
Figures


Similar articles
-
Evolution of an extreme hemoglobin phenotype contributed to the sub-Arctic specialization of extinct Steller's sea cows.Elife. 2023 Jun 1;12:e85414. doi: 10.7554/eLife.85414. Elife. 2023. PMID: 37259901 Free PMC article.
-
Using the MWC model to describe heterotropic interactions in hemoglobin.PLoS One. 2017 Aug 9;12(8):e0182871. doi: 10.1371/journal.pone.0182871. eCollection 2017. PLoS One. 2017. PMID: 28793329 Free PMC article.
-
Oxygen binding by single crystals of hemoglobin: the problem of cooperativity and inequivalence of alpha and beta subunits.Proteins. 1996 Aug;25(4):425-37. doi: 10.1002/prot.3. Proteins. 1996. PMID: 8865338
-
How does hemoglobin generate such diverse functionality of physiological relevance?Biochim Biophys Acta. 2013 Sep;1834(9):1873-84. doi: 10.1016/j.bbapap.2013.04.026. Epub 2013 May 1. Biochim Biophys Acta. 2013. PMID: 23643742 Review.
-
Molecular adaptation of hemoglobin function in mammals.Respir Physiol. 1992 Oct;90(1):3-17. doi: 10.1016/0034-5687(92)90130-o. Respir Physiol. 1992. PMID: 1455096 Review.
Cited by
-
Quantitative dissection of transcription in development yields evidence for transcription-factor-driven chromatin accessibility.Elife. 2020 Oct 19;9:e56429. doi: 10.7554/eLife.56429. Elife. 2020. PMID: 33074101 Free PMC article.
References
-
- Bunn H-F. Regulation of hemoglobin function in mammals. American Zoologist. 1980;20:199–211. doi: 10.1093/icb/20.1.199. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

