Assessing direct and indirect effects of pediatric influenza vaccination in Germany by individual-based simulations
- PMID: 31647348
- PMCID: PMC7227695
- DOI: 10.1080/21645515.2019.1682843
Assessing direct and indirect effects of pediatric influenza vaccination in Germany by individual-based simulations
Abstract
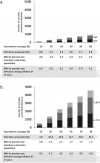
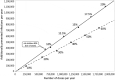
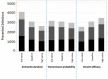
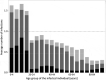
Children have a high burden of influenza and play a central role in spreading influenza. Routinely vaccinating children against influenza may, thus, not only reduce their disease burden, but also that of the general population, including the elderly who frequently suffer severe complications. Using the published individual-based tool 4Flu, we simulated how pediatric vaccination would change infection incidence in Germany. Transmission of four influenza strains was simulated in 100,000 individuals with German demography and contact structure. After initialization with the recorded trivalent influenza vaccination coverage for 20 years (1997-2016), all vaccinations were switched to quadrivalent influenza vaccine (QIV). Scenarios where vaccination coverage of children (0.5-17-year-old) was increased from the current value (4.3%) to a maximum of 10-60% were compared to baseline with unchanged coverage, averaging results of 1,000 pairs of simulations over a 20-year evaluation period (2017-2036). Pediatric vaccination coverage of 10-60% annually prevented 218-1,732 (6.3-50.5%) infections in children, 204-1,961 (2.9-28.2%) in young adults and 95-868 (3.1-28.9%) in the elderly in a population of 100,000 inhabitants; overall, 34.1% of infections in the total population (3.7 million infections per year in Germany) can be prevented if 60% of all children are vaccinated annually. 4.4-4.6 vaccinations were needed to prevent one infection among children; 1.7-1.8 were needed to prevent one in the population. Enhanced pediatric vaccination prevents many infections in children and even more in young adults and the elderly.
Keywords: Influenza; mathematical model; pediatric; simulation; vaccination.
Figures
References
-
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010;2:CD004876. - PubMed
-
- Demicheli V, Jefferson T, Al-Ansary LA, Ferroni E, Rivetti A, Di PC. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2014;3:CD001269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
