A unicellular relative of animals generates a layer of polarized cells by actomyosin-dependent cellularization
- PMID: 31647412
- PMCID: PMC6855841
- DOI: 10.7554/eLife.49801
A unicellular relative of animals generates a layer of polarized cells by actomyosin-dependent cellularization
Erratum in
-
Correction: A unicellular relative of animals generates a layer of polarized cells by actomyosin-dependent cellularization.Elife. 2020 Jun 19;9:e60055. doi: 10.7554/eLife.60055. Elife. 2020. PMID: 32553113 Free PMC article.
Abstract
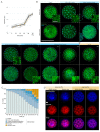
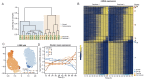
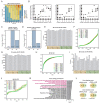
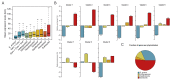
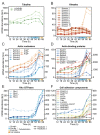
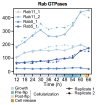
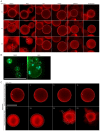
In animals, cellularization of a coenocyte is a specialized form of cytokinesis that results in the formation of a polarized epithelium during early embryonic development. It is characterized by coordinated assembly of an actomyosin network, which drives inward membrane invaginations. However, whether coordinated cellularization driven by membrane invagination exists outside animals is not known. To that end, we investigate cellularization in the ichthyosporean Sphaeroforma arctica, a close unicellular relative of animals. We show that the process of cellularization involves coordinated inward plasma membrane invaginations dependent on an actomyosin network and reveal the temporal order of its assembly. This leads to the formation of a polarized layer of cells resembling an epithelium. We show that this stage is associated with tightly regulated transcriptional activation of genes involved in cell adhesion. Hereby we demonstrate the presence of a self-organized, clonally-generated, polarized layer of cells in a unicellular relative of animals.
Keywords: actin; cell biology; cellularization; coenocyte; epithelium; evolution; evolutionary biology; multicellularity.
© 2019, Dudin et al.
Conflict of interest statement
OD, AO, XG, AH, AT, HS, JB, IR No competing interests declared
Figures






Comment in
-
How contraction has shaped evolution.Elife. 2019 Nov 14;8:e52805. doi: 10.7554/eLife.52805. Elife. 2019. PMID: 31724951 Free PMC article.
References
-
- Afshar K, Stuart B, Wasserman SA. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 2000;127:1887–1897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Consolidator Grant ERC-2012-Co-616960/ERC_/European Research Council/International
- MEXT KAKENHI 221S0002/Ministry of Education, Culture, Sports, Science and Technology/International
- MEXT KAKENHI 26891021/Ministry of Education, Culture, Sports, Science and Technology/International
- Young Research Talents grant 240284/Research Council of Norway/International
- P2LAP3_171815/SNSF_/Swiss National Science Foundation/Switzerland
- Individual fellowship MSCA-IF 746044/H2020 Marie Skłodowska-Curie Actions/International
- Individual fellowship MSCA-IF 747086/H2020 Marie Skłodowska-Curie Actions/International
- ERC-2012-Co -616960/European Research Council Consolidator Grant/International
- 221S0002/MEXT KAKENHI/International
- 26891021/MEXT KAKENHI/International
- 240284/Young Research Talents grant from the Research Council of Norway/International
- MSCA-IF 746044/Marie Sklodowska-Curie individual fellowship/International
- MSCA-IF 747086/Marie Sklodowska-Curie individual fellowship/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

