Functional Integration of Neuronal Precursors in the Adult Murine Piriform Cortex
- PMID: 31647533
- PMCID: PMC7132906
- DOI: 10.1093/cercor/bhz181
Functional Integration of Neuronal Precursors in the Adult Murine Piriform Cortex
Abstract
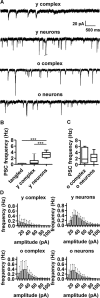
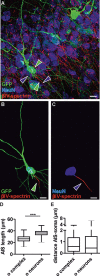
The extent of functional maturation and integration of nonproliferative neuronal precursors, becoming neurons in the adult murine piriform cortex, is largely unexplored. We thus questioned whether precursors eventually become equivalent to neighboring principal neurons or whether they represent a novel functional network element. Adult brain neuronal precursors and immature neurons (complex cells) were labeled in transgenic mice (DCX-DsRed and DCX-CreERT2 /flox-EGFP), and their cell fate was characterized with patch clamp experiments and morphometric analysis of axon initial segments. Young (DCX+) complex cells in the piriform cortex of 2- to 4-month-old mice received sparse synaptic input and fired action potentials at low maximal frequency, resembling neonatal principal neurons. Following maturation, the synaptic input detected on older (DCX-) complex cells was larger, but predominantly GABAergic, despite evidence of glutamatergic synaptic contacts. Furthermore, the rheobase current of old complex cells was larger and the maximal firing frequency was lower than those measured in neighboring age-matched principal neurons. The striking differences between principal neurons and complex cells suggest that the latter are a novel type of neuron and new coding element in the adult brain rather than simple addition or replacement for preexisting network components.
Keywords: adult neurogenesis; axon initial segment; complex cells; doublecortin; tangled cells.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures






References
-
- Alyan SH. 1996. Evidence for resetting the directional component of path integration in the house mouse (Mus musculus). Ethology. 102:629–638.
-
- Alyan SH, Jander R. 1994. Short-range homing in the house mouse, Mus musculus: stages in the learning of directions. Anim Behav. 48(2):285–298.
-
- Arellano JI, Espinosa A, Fairén A. 2007. Non-synaptic dendritic spines in neocortex. Neuroscience. 145:464–469. - PubMed
-
- Ben-Ari Y. 2002. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 3:728–739. - PubMed
-
- Bhatt DH, Zhang S, Gan W-B. 2009. Dendritic spine dynamics. Annu Rev Physiol. 71:261–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

