Chlamydia trachomatis recruits protein kinase C during infection
- PMID: 31647538
- PMCID: PMC7056930
- DOI: 10.1093/femspd/ftz061
Chlamydia trachomatis recruits protein kinase C during infection
Abstract
Chlamydia trachomatis is a significant pathogen with global and economic impact. As an obligate intracellular pathogen, C. trachomatis resides inside the inclusion, a parasitophorous vacuole, and depends on the host cell for survival and transition through a biphasic development cycle. During infection, C. trachomatis is known to manipulate multiple signaling pathways and recruit an assortment of host proteins to the inclusion membrane, including host kinases. Here, we show recruitment of multiple isoforms of protein kinase C (PKC) including active phosphorylated PKC isoforms to the chlamydial inclusion colocalizing with active Src family kinases. Pharmacological inhibition of PKC led to a modest reduction of infectious progeny production. PKC phosphorylated substrates were seen recruited to the entire periphery of the inclusion membrane. Infected whole cell lysates showed altered PKC phosphorylation of substrates during the course of infection. Assessment of different chlamydial species showed recruitment of PKC and PKC phosphorylated substrates were limited to C. trachomatis. Taken together, PKC and PKC substrate recruitment may provide significant insights into how C. trachomatis manipulates multiple host signaling cascades during infection.
Keywords: Chlamydia; microdomains; phosphorylation; protein kinase C.
© FEMS 2019.
Figures
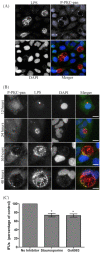
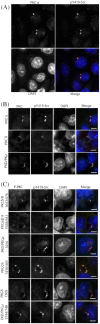
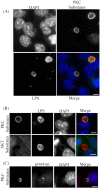
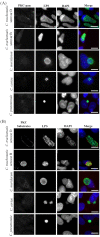
Similar articles
-
A meta-analysis of affinity purification-mass spectrometry experimental systems used to identify eukaryotic and chlamydial proteins at the Chlamydia trachomatis inclusion membrane.J Proteomics. 2020 Feb 10;212:103595. doi: 10.1016/j.jprot.2019.103595. Epub 2019 Nov 21. J Proteomics. 2020. PMID: 31760040 Free PMC article.
-
Recruitment of BAD by the Chlamydia trachomatis vacuole correlates with host-cell survival.PLoS Pathog. 2006 May;2(5):e45. doi: 10.1371/journal.ppat.0020045. Epub 2006 May 19. PLoS Pathog. 2006. PMID: 16710454 Free PMC article.
-
The Human Centrosomal Protein CCDC146 Binds Chlamydia trachomatis Inclusion Membrane Protein CT288 and Is Recruited to the Periphery of the Chlamydia-Containing Vacuole.Front Cell Infect Microbiol. 2018 Jul 26;8:254. doi: 10.3389/fcimb.2018.00254. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30094225 Free PMC article.
-
Chlamydial intracellular survival strategies.Cold Spring Harb Perspect Med. 2013 May 1;3(5):a010256. doi: 10.1101/cshperspect.a010256. Cold Spring Harb Perspect Med. 2013. PMID: 23637308 Free PMC article. Review.
-
Autophagy: the misty lands of Chlamydia trachomatis infection.Front Cell Infect Microbiol. 2024 Sep 6;14:1442995. doi: 10.3389/fcimb.2024.1442995. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39310786 Free PMC article. Review.
Cited by
-
The Small Molecule H89 Inhibits Chlamydia Inclusion Growth and Production of Infectious Progeny.Infect Immun. 2021 Jun 16;89(7):e0072920. doi: 10.1128/IAI.00729-20. Epub 2021 Jun 16. Infect Immun. 2021. PMID: 33820812 Free PMC article.
-
Hijacking and Use of Host Kinases by Chlamydiae.Pathogens. 2020 Dec 10;9(12):1034. doi: 10.3390/pathogens9121034. Pathogens. 2020. PMID: 33321710 Free PMC article. Review.
-
An Update on Protein Kinases as Therapeutic Targets-Part I: Protein Kinase C Activation and Its Role in Cancer and Cardiovascular Diseases.Int J Mol Sci. 2023 Dec 18;24(24):17600. doi: 10.3390/ijms242417600. Int J Mol Sci. 2023. PMID: 38139428 Free PMC article. Review.
References
-
- Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol Rev. 2005;29:949–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

