Efficacy of a Texting Program to Promote Cessation Among Pregnant Smokers: A Randomized Control Trial
- PMID: 31647564
- PMCID: PMC7291805
- DOI: 10.1093/ntr/ntz174
Efficacy of a Texting Program to Promote Cessation Among Pregnant Smokers: A Randomized Control Trial
Abstract
Introduction: Smoking during pregnancy poses serious risks to baby and mother. Few disseminable programs exist to help pregnant women quit or reduce their smoking. We hypothesized that an SMS text-delivered scheduled gradual reduction (SGR) program plus support texts would outperform SMS support messages alone.
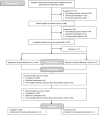
Methods: We recruited 314 pregnant women from 14 prenatal clinics. Half of the women received theory-based support messages throughout their pregnancy to promote cessation and prevent relapse. The other half received the support messages plus alert texts that gradually reduced their smoking more than 3-5 weeks. We conducted surveys at baseline, end of pregnancy, and 3 months postpartum. Our primary outcome was biochemically validated 7-day point prevalence abstinence at late pregnancy. Our secondary outcome was reduction in cigarettes per day.
Results: Adherence to the SGR was adequate with 70% responding to alert texts to smoke within 60 minutes. Women in both arms quit smoking at the same rate (9%-12%). Women also significantly reduced their smoking from baseline to the end of pregnancy from nine cigarettes to four; we found no arm differences in reduction.
Conclusions: Support text messages alone produced significant quit rates above naturally occurring quitting. SGR did not add significantly to helping women quit or reduce. Sending support messages can reach many women and is low-cost. More obstetric providers might consider having patients who smoke sign up for free texting programs to help them quit.
Implications: A disseminable texting program helped some pregnant women quit smoking.Clinical Trial Registration number: NCT01995097.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
A pilot study testing SMS text delivered scheduled gradual reduction to pregnant smokers.Nicotine Tob Res. 2013 Oct;15(10):1773-6. doi: 10.1093/ntr/ntt045. Epub 2013 Apr 8. Nicotine Tob Res. 2013. PMID: 23569007 Free PMC article. Clinical Trial.
-
Pilot Test of Connecting Pregnant Women who Smoke to Short Message Service (SMS) Support Texts for Cessation.Matern Child Health J. 2020 Apr;24(4):419-422. doi: 10.1007/s10995-020-02893-8. Matern Child Health J. 2020. PMID: 32026323
-
Peer Mentoring and Automated Text Messages for Smoking Cessation: A Randomized Pilot Trial.Nicotine Tob Res. 2020 Mar 16;22(3):371-380. doi: 10.1093/ntr/ntz047. Nicotine Tob Res. 2020. PMID: 30892616 Clinical Trial.
-
[Smoking reduction and temporary abstinence: new approaches for smoking cessation].J Mal Vasc. 2003 Dec;28(5):293-300. J Mal Vasc. 2003. PMID: 14978435 Review. French.
-
[Review about smoking in pregnancy: prevalence, sociodemographic profile, perinatal depression, psychological variables involved and treatment].Rev Esp Salud Publica. 2024 Oct 11;98:e202410055. Rev Esp Salud Publica. 2024. PMID: 39391965 Review. Spanish.
Cited by
-
Effectiveness evaluation of an organisational intervention, targeting pregnancy and addiction care professionals, among women who have just given birth in maternity wards and smoked tobacco during pregnancy (5A-QUIT-N): study protocol for a stepped-wedge cluster randomised trial.BMJ Open. 2024 Nov 12;14(11):e087541. doi: 10.1136/bmjopen-2024-087541. BMJ Open. 2024. PMID: 39532364 Free PMC article.
-
Technology-Based Interventions in Tobacco Use Treatment Among People Who Identify as African American/Black, Hispanic/Latina/o, and American Indian/Alaska Native: Scoping Review.J Med Internet Res. 2024 Oct 10;26:e50748. doi: 10.2196/50748. J Med Internet Res. 2024. PMID: 39388699 Free PMC article.
-
Trajectories of Situational Temptations in Pregnant Smokers participating in a Scheduled Gradual Reduction Cessation Trial.Matern Child Health J. 2022 Jan;26(1):24-30. doi: 10.1007/s10995-021-03321-1. Epub 2021 Dec 3. Matern Child Health J. 2022. PMID: 34860350 Free PMC article. Clinical Trial.
-
Reducing Smoking Cessation Disparities: Capacity for a Primary Care- and Technology-Based Approach Among Medicaid Recipients.J Clin Psychol Med Settings. 2023 Sep;30(3):636-644. doi: 10.1007/s10880-022-09925-1. Epub 2022 Nov 18. J Clin Psychol Med Settings. 2023. PMID: 36400987
-
Effect of digital health, biomarker feedback and nurse or midwife-led counselling interventions to assist pregnant smokers quit: a systematic review and meta-analysis.BMJ Open. 2023 Mar 24;13(3):e060549. doi: 10.1136/bmjopen-2021-060549. BMJ Open. 2023. PMID: 36963792 Free PMC article.
References
-
- Goldenberg RL, Davis RO, Cliver SP, et al. . Maternal risk factors and their influence on fetal anthropometric measurements. Am J Obstet Gynecol. 1993;168(4):1197–1203; discussion 1203. - PubMed
-
- Chapin J, Root W; American College of Obstetricians and Gynecologists Improving obstetrician-gynecologist implementation of smoking cessation guidelines for pregnant women: an interim report of the American College of Obstetricians and Gynecologists. Nicotine Tob Res. 2004;6(suppl 2):S253–S257. - PubMed
-
- Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16(3):208–215. - PubMed
-
- Salihu HM, Aliyu MH, Pierre-Louis BJ, Alexander GR. Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J. 2003;7(4):219–227. - PubMed
-
- Centers for Disease Control and Prevention. Smoking during pregnancy—United States, 1990–2002. MMWR Morb Mortal Wkly Rep. 2004;53(39):911–915. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical