Endothelial cell-specific redox gene modulation inhibits angiogenesis but promotes B16F0 tumor growth in mice
- PMID: 31647879
- PMCID: PMC6894059
- DOI: 10.1096/fj.201900786R
Endothelial cell-specific redox gene modulation inhibits angiogenesis but promotes B16F0 tumor growth in mice
Abstract
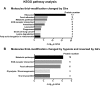
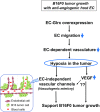
Glutaredoxin-1 (Glrx) is a small cytosolic enzyme that removes S-glutathionylation, glutathione adducts of protein cysteine residues, thus modulating redox signaling and gene transcription. Although Glrx up-regulation prevented endothelial cell (EC) migration and global Glrx transgenic mice had impaired ischemic vascularization, the effects of cell-specific Glrx overexpression remained unknown. Here, we examined the role of EC-specific Glrx up-regulation in distinct models of angiogenesis; namely, hind limb ischemia and tumor angiogenesis. EC-specific Glrx transgenic (EC-Glrx TG) overexpression in mice significantly impaired EC migration in Matrigel implants and hind limb revascularization after femoral artery ligation. Additionally, ECs migrated less into subcutaneously implanted B16F0 melanoma tumors as assessed by decreased staining of EC markers. Despite reduced angiogenesis, EC-Glrx TG mice unexpectedly developed larger tumors compared with control mice. EC-Glrx TG mice showed higher levels of VEGF-A in the tumors, indicating hypoxia, which may stimulate tumor cells to form vascular channels without EC, referred to as vasculogenic mimicry. These data suggest that impaired ischemic vascularization does not necessarily associate with suppression of tumor growth, and that antiangiogenic therapies may be ineffective for melanoma tumors because of their ability to implement vasculogenic mimicry during hypoxia.-Yura, Y., Chong, B. S. H., Johnson, R. D., Watanabe, Y., Tsukahara, Y., Ferran, B., Murdoch, C. E., Behring, J. B., McComb, M. E., Costello, C. E., Janssen-Heininger, Y. M. W., Cohen, R. A., Bachschmid, M. M., Matsui, R. Endothelial cell-specific redox gene modulation inhibits angiogenesis but promotes B16F0 tumor growth in mice.
Keywords: S-glutathionylation; glutaredoxin; melanoma; vascular; vasculogenic mimicry.
Conflict of interest statement
The authors thank Dr. Nader Rahimi for advice, and Dominique Croteau for editing the manuscript (both from the Boston University School of Medicine). The authors also thank Professor Stephen Chlopicki and his laboratory members (Jagiellonian Centre for Experimental Therapeutics, Krakow, Poland) for helpful advice, and the Analytical Instrumentation Core at Boston University Medical Campus for their service. This research was supported by U.S. National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL133013 (to R.M.), postdoctoral training Grant T32 HL70024 (to B.F.), R37 HL104017 (to R.A.C.), and contract HHSN268201000031C (to C.E.C.); NIH/National Institute on Aging Grant R03 AG051857 (to R.M.); NIH/National Center for Advancing Translational Sciences Grant 1UL1 TR001430 (to M.M.B. and R.M.); NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK103750 (to M.M.B.); and American Heart Association Grant 16GRNT27660006 (to M.M.B.). The authors declare no conflicts of interest.
Figures





References
-
- Sundaresan M., Yu Z. X., Ferrans V. J., Irani K., Finkel T. (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 - PubMed
-
- Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. (2009) Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem. Sci. 34, 85–96 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

