MANET 3.0: Hierarchy and modularity in evolving metabolic networks
- PMID: 31648227
- PMCID: PMC6812854
- DOI: 10.1371/journal.pone.0224201
MANET 3.0: Hierarchy and modularity in evolving metabolic networks
Abstract
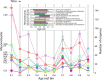
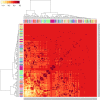
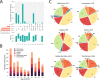
Enzyme recruitment is a fundamental evolutionary driver of modern metabolism. We see evidence of recruitment at work in the metabolic Molecular Ancestry Networks (MANET) database, an online resource that integrates data from KEGG, SCOP and structural phylogenomic reconstruction. The database, which was introduced in 2006, traces the deep history of the structural domains of enzymes in metabolic pathways. Here we release version 3.0 of MANET, which updates data from KEGG and SCOP, links enzyme and PDB information with PDBsum, and traces evolutionary information of domains defined at fold family level of SCOP classification in metabolic subnetwork diagrams. Compared to SCOP folds used in the previous versions, fold families are cohesive units of functional similarity that are highly conserved at sequence level and offer a 10-fold increase of data entries. We surveyed enzymatic, functional and catalytic site distributions among superkingdoms showing that ancient enzymatic innovations followed a biphasic temporal pattern of diversification typical of module innovation. We grouped enzymatic activities of MANET into a hierarchical system of subnetworks and mesonetworks matching KEGG classification. The evolutionary growth of these modules of metabolic activity was studied using bipartite networks and their one-mode projections at enzyme, subnetwork and mesonetwork levels of organization. Evolving metabolic networks revealed patterns of enzyme sharing that transcended mesonetwork boundaries and supported the patchwork model of metabolic evolution. We also explored the scale-freeness, randomness and small-world properties of evolving networks as possible organizing principles of network growth and diversification. The network structure shows an increase in hierarchical modularity and scale-free behavior as metabolic networks unfold in evolutionary time. Remarkably, this evolutionary constraint on structure was stronger at lower levels of metabolic organization. Evolving metabolic structure reveals a 'principle of granularity', an evolutionary increase of the cohesiveness of lower-level parts of a hierarchical system. MANET is available at http://manet.illinois.edu.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures












References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

