Molecular response with blinatumomab in relapsed/refractory B-cell precursor acute lymphoblastic leukemia
- PMID: 31648325
- PMCID: PMC6849936
- DOI: 10.1182/bloodadvances.2019000457
Molecular response with blinatumomab in relapsed/refractory B-cell precursor acute lymphoblastic leukemia
Abstract
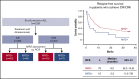
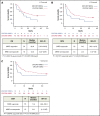
Minimal residual disease (MRD), where leukemic cell levels are lower than the morphologic detection threshold, is the most important prognostic factor for acute lymphoblastic leukemia (ALL) relapse during first-line chemotherapy treatment and is standard of care in treatment monitoring and decision making. Limited data are available on the prognostic value of MRD response after relapse. We evaluated the relationship between MRD response and outcomes in blinatumomab-treated adults with relapsed/refractory (R/R) B-cell precursor ALL. Of 90 patients with complete remission (CR) or CR with partial hematologic recovery (CRh), 64 (71.1%) achieved a complete MRD response (no detectable individual rearrangements of immunoglobulin/T-cell receptor genes by polymerase chain reaction [PCR] at a minimum sensitivity level of 10-4). Eleven patients had MRD <10-4. Therefore, overall, 75 (83.3%) experienced an MRD response (no detectable MRD or detectable MRD) measured by PCR within the first 2 treatment cycles. Overall survival (OS) and relapse-free survival (RFS) were significantly longer in patients who achieved CR/CRh and MRD response (median, 20.6 and 9.0 months, respectively) compared with CR/CRh patients without MRD response (median, 12.5 and 2.3 months, respectively). In conclusion, longer durations of OS and RFS associated with MRD response support the value of achieving MRD response and its use as a prognostic factor for blinatumomab treatment in R/R ALL. This trial was registered at www.clinicaltrials.gov as #NCT01466179.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: N.G. received research funding from Amgen, Pfizer, and Novartis; received advisory board and speaker fees from Amgen, Pfizer, and Novartis; received travel grants from Amgen, Pfizer, and Novartis; and received advisory board honoraria from Kite/Gilead and Celgene. H.M.K. received research funding from AbbVie, Agios, Amgen, Ariad, Astex, Bristol-Myers Squibb, Cyclacel, ImmunoGen, Jazz, and Pfizer and received honoraria from AbbVie, Actinium, Agios, Amgen, ImmunoGen, Orsinex, Pfizer, and Takeda. M.B. received research funding from Amgen; received reference diagnostics from Affimed, Amgen, and Regeneron; received consulting fees from PRMA Consulting, Ltd; and received speaker fees from Amgen, Hoffman-La Roche, Incyte, and Pfizer. A.S.S. received speaker fees from Amgen and Celgene. R.C.B. received fees for advisory activities from Amgen, AstraZeneca, Cellex, GEMoaB Monoclonals, GmbH, Molecular Partners, Novartis, and Pfizer; and has a blinatumomab patent with royalties paid. H.D. received research funding from Amgen, Incyte/Ariad, Novartis, Pfizer, and Servier; received consultancy fees from Amgen, Celgene, Cellectis, and Pfizer; received advisor honoraria from Amgen, Celgene, Cellectis, Incyte/Ariad, Novartis, Pfizer, Servier, and Shire/Baxalta; and received speaker fees from Amgen, Celgene, Incyte/Ariad, and Pfizer. L.H. received institutional research funding from AbbVie, ADC Therapeutics, Astex Pharmaceuticals, Genentech, and Pharmacyclics and received personal fees from Pharmacyclics. F.R.-H. received advisory board fees from Amgen, Bristol-Myers Squibb, Incyte, Jazz Pharmaceuticals, Novartis, and Pfizer. M.L. received research funding from Amgen. S.O. received consultancy fees from Amgen. G.Z. is an employee of Amgen and holds several pending patents. S.G. is a former employee of Amgen. D.N. is an employee and stockholder of Amgen and holds blinatumomab-related pending patents. M.S.T. received research funding and personal fees from Amgen. The remaining authors declare no competing financial interests.
Figures
References
-
- Dombret H, Cluzeau T, Huguet F, Boissel N. Pediatric-like therapy for adults with ALL. Curr Hematol Malig Rep. 2014;9(2):158-164. - PubMed
-
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532-543. - PubMed
-
- National Comprehensive Cancer Network NCCN Guidelines Acute Lymphoblastic Leukemia v2.2014 Fort Washington, PA: National Comprehensive Cancer Network; 2014.
-
- Oriol A, Vives S, Hernández-Rivas JM, et al. ; Programa Español de Tratamiento en Hematologia Group . Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95(4):589-596. - PMC - PubMed
-
- Tavernier E, Boiron JM, Huguet F, et al. ; GET-LALA Group; Swiss Group for Clinical Cancer Research SAKK; Australasian Leukaemia and Lymphoma Group . Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907-1914. - PubMed

