Safety of allogeneic hematopoietic cell transplant in adults after CD19-targeted CAR T-cell therapy
- PMID: 31648329
- PMCID: PMC6849954
- DOI: 10.1182/bloodadvances.2019000593
Safety of allogeneic hematopoietic cell transplant in adults after CD19-targeted CAR T-cell therapy
Abstract
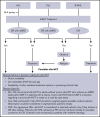
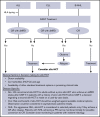
Allogeneic hematopoietic cell transplantation (allo-HCT) is offered to selected patients after chimeric antigen receptor-modified T-cell (CAR-T) therapy. Lymphodepleting chemotherapy and CAR-T therapy have immunosuppressive and immunomodulatory effects that could alter the safety profile of subsequent allo-HCT. We reviewed our experience with 32 adults (acute lymphoblastic leukemia [ALL], n = 19; B-cell non-Hodgkin lymphoma [NHL]/chronic lymphocytic leukemia [CLL], n = 13) who received an allo-HCT after CAR-T therapy, with a focus on posttransplant toxicities. Myeloablative conditioning (MAC) was used in 74% of ALL patients and 39% of NHL/CLL patients. The median time from CAR-T therapy to allo-HCT was 72 days in ALL patients and 122 days in NHL/CLL patients. Cumulative incidences of grade 3-4 acute graft-versus-host disease (GVHD) and chronic GVHD were 25% and 10%, respectively. All patients had neutrophil recovery (median, 18.5 days) and all but 3 had platelet recovery (median, 12 days). Twenty-two percent had viral or systemic fungal infection within 100 days after allo-HCT. The 100-day and 1-year cumulative incidences of NRM were 16% and 21%, respectively, for ALL patients and 15% and 33%, respectively, for NHL/CLL patients. In ALL patients, later utilization of allo-HCT after CAR-T therapy was associated with higher mortality. In NHL/CLL patients, MAC was associated with higher mortality. Toxicities did not exceed the expected incidences in this high-risk population.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.S. has acted as a consultant or played an advisory role for AbbVie, Genentech, AstraZeneca, Sound Biologics, Verastem Oncology, ADC Therapeutics, Pharmacyclics, and Atara Biotherapeutics and has received research funding from Mustang Biopharma, Celgene, Pharmacyclics, Gilead Sciences, Genentech, AbbVie, TG Therapeutics, BeiGene, Acerta Pharma, Merck, and Sunesis. R.D.C. has received research funding from Amgen, Incyte, Kite/Gilead, Merck, Pfizer, and Vanda Pharmaceuticals and has served as a consultant/advisor to Amgen and Pfizer. B.G.T. has received research funding and patent/royalties from Mustang Bio. A.K.G. has received grants and nonfinancial support from Teva Pharmaceutical Industries, Bristol-Myers Squibb, Merck, Takeda, TG Therapeutics, and Effector; has received grants, personal fees, and nonfinancial support from Seattle Genetics, Pfizer, Janssen, Gilead Sciences, Spectrum, Amgen, and Incyte; and has received personal fees from Aptevo Therapeutics, BRIM Biotechnology, Seattle Genetics, Amgen, Acerta, I-Mab Biopharma, and Sanofi. B.M.S. has acted as a consultant for Kiadis Pharma, Actinium Pharmaceuticals, Bristol-Meyers Squibb, and Frazier Healthcare Ventures; has received research funding from Bellicum Pharmaceuticals; has equity ownership in AnaptysBio, OncoResponse, Inipharm, Mavupharma Inc., EpiThany, and Blaze Bioscience; and has been employed by Mavupharma Inc. D.G.M. has received honoraria from Kite Pharma, Gilead Sciences, Celgene, Genentech, and Novartis; has received institutional research funding from Kite Pharma, Juno Therapeutics, and Celgene; and has received remuneration for travel and accommodations from A2 Biotherapeutics. C.J.T. has received research funding from Juno Therapeutics, a Celgene company, and Nektar Therapeutics; holds a patent licensed to Juno Therapeutics, a Celgene company; has served on advisory boards and has options in Caribou Biosciences, Eureka Therapeutics, and Precision Biosciences; and has served on advisory boards for Aptevo, Humanigen, Juno Therapeutics, a Celgene company, Kite Pharma, a Gilead Company, Nektar Therapeutics, Novartis, T-CURX, and Allogene Therapeutics. The remaining authors declare no competing financial interests.
Figures

References
-
- Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. - PubMed

