Cell Fusion: Merging Membranes and Making Muscle
- PMID: 31648852
- PMCID: PMC7849503
- DOI: 10.1016/j.tcb.2019.09.002
Cell Fusion: Merging Membranes and Making Muscle
Abstract
Cell fusion is essential for the development of multicellular organisms, and plays a key role in the formation of various cell types and tissues. Recent findings have highlighted the varied protein machinery that drives plasma-membrane merger in different systems, which is characterized by diverse structural and functional elements. We highlight the discovery and activities of several key sets of fusion proteins that together offer an evolving perspective on cell membrane fusion. We also emphasize recent discoveries in vertebrate myoblast fusion in skeletal muscle, which is composed of numerous multinucleated myofibers formed by the fusion of progenitor cells during development.
Keywords: cell fusion; fusogens; myoblast fusion; myomaker; myomerger; skeletal muscle.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

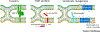

References
-
- Chal J and Pourquie O (2017) Making muscle: skeletal myogenesis in vivo and in vitro. Development 144, 2104–2122 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

