PACAP and Other Neuropeptide Targets Link Chronic Migraine and Opioid-induced Hyperalgesia in Mouse Models
- PMID: 31649062
- PMCID: PMC6885698
- DOI: 10.1074/mcp.RA119.001767
PACAP and Other Neuropeptide Targets Link Chronic Migraine and Opioid-induced Hyperalgesia in Mouse Models
Abstract
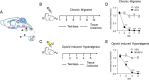
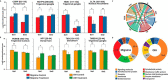
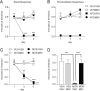
Chronic use of opioids can produce opioid-induced hyperalgesia (OIH), and when used to treat migraine, these drugs can result in increased pain and headache chronicity. We hypothesized that overlapping mechanisms between OIH and chronic migraine occur through neuropeptide dysregulation. Using label-free, non-biased liquid chromatography-mass spectrometry to identify and measure changes in more than 1500 neuropeptides under these two conditions, we observed only 16 neuropeptides that were altered between the two conditions. The known pro-migraine molecule, calcitonin-gene related peptide, was among seven peptides associated with chronic migraine, with several pain-processing neuropeptides among the nine other peptides affected in OIH. Further, composite peptide complements Pituitary adenylate cyclase-activating polypeptide (PACAP), Vasoactive intestinal peptide (VIP) and Secretogranin (SCG) showed significant changes in both chronic migraine and OIH. In a follow-up pharmacological study, we confirmed the role of PACAP in models of these two disorders, validating the effectiveness of our peptidomic approach, and identifying PACAP as a mechanistic link between chronic migraine and OIH. Data are available via ProteomeXchange with identifier PXD013362.
Keywords: Peptides; animal models; headache; hyperalgesia; mass spectrometry; mouse models; neurobiology*; pain; peptidomics.
© 2019 Anapindi et al.
Figures

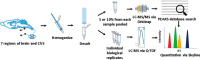

References
-
- Charles A. Migraine. N. Engl. J. Med. 2017;377:553–561. - PubMed
-
- Bigal M.E., Lipton R.B. Excessive opioid use and the development of chronic migraine. Pain. 2009;142:179–182. - PubMed
-
- Buse D.C., Pearlman S.H., Reed M.L., Serrano D., Ng-Mak D.S., Lipton R.B. Opioid use and dependence among persons with migraine: results of the AMPP study. Headache. 2012;52:18–36. - PubMed
-
- Hayhurst C.J., Durieux M.E. Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology. 2016;124:483–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

