Tests of the chromatographic theory of olfaction with highly soluble odors: a combined electro-olfactogram and computational fluid dynamics study in the mouse
- PMID: 31649069
- PMCID: PMC6826284
- DOI: 10.1242/bio.047217
Tests of the chromatographic theory of olfaction with highly soluble odors: a combined electro-olfactogram and computational fluid dynamics study in the mouse
Abstract
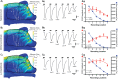
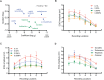
The idea that the vertebrate nasal cavity operates like a gas chromatograph to separate and discriminate odors, referred to herein as the 'chromatographic theory' (CT), has a long and interesting history. Though the last decade has seen renewed interest in the notion, its validity remains in question. Here we examine a necessary condition of the theory: a correlation between nasal odor deposition patterns based on mucus solubility and the distribution of olfactory sensory neuron odotypes. Our recent work in the mouse failed to find such a relationship even across large sorption gradients within the olfactory epithelium (OE). However, these studies did not test extremely soluble odorants or low odor concentrations, factors that could explain our inability to find supporting evidence for the CT. The current study combined computational fluid dynamics (CFD) simulations of odor sorption patterns and electro-olfactogram (EOG) measurements of olfactory sensory neuron responses. The odorants tested were at the extremes of mucus solubility and at a range of concentrations. Results showed no relationship between local odor sorption patterns and EOG response maps. Together, results again failed to support a necessary condition of the CT casting further doubt on the viability of this classical odor coding mechanism.
Keywords: Maps; Odor discrimination; Olfactory receptors; Sorption.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Tests of the sorption and olfactory "fovea" hypotheses in the mouse.J Neurophysiol. 2017 Nov 1;118(5):2770-2788. doi: 10.1152/jn.00455.2017. Epub 2017 Sep 6. J Neurophysiol. 2017. PMID: 28877965 Free PMC article.
-
Tuning to odor solubility and sorption pattern in olfactory epithelial responses.J Neurosci. 2014 Feb 5;34(6):2025-36. doi: 10.1523/JNEUROSCI.3736-13.2014. J Neurosci. 2014. PMID: 24501345 Free PMC article.
-
An electroolfactogram study of odor response patterns from the mouse olfactory epithelium with reference to receptor zones and odor sorptiveness.J Neurophysiol. 2013 Apr;109(8):2179-91. doi: 10.1152/jn.00769.2012. Epub 2013 Jan 23. J Neurophysiol. 2013. PMID: 23343905
-
Olfaction in fish.Prog Neurobiol. 1975;5(4):271-335. doi: 10.1016/0301-0082(75)90014-3. Prog Neurobiol. 1975. PMID: 830087 Review.
-
Spatial patterning of response to odors in the peripheral olfactory system.Physiol Rev. 1976 Jul;56(3):578-93. doi: 10.1152/physrev.1976.56.3.578. Physiol Rev. 1976. PMID: 778869 Review.
Cited by
-
Odor coding in the mammalian olfactory epithelium.Cell Tissue Res. 2021 Jan;383(1):445-456. doi: 10.1007/s00441-020-03327-1. Epub 2021 Jan 6. Cell Tissue Res. 2021. PMID: 33409650 Free PMC article. Review.
-
Establishment of an Olfactory Region-specific Intranasal Delivery Technique in Mice to Target the Central Nervous System.Front Pharmacol. 2022 Jan 10;12:789780. doi: 10.3389/fphar.2021.789780. eCollection 2021. Front Pharmacol. 2022. PMID: 35082672 Free PMC article.
-
A 3D transcriptomics atlas of the mouse nose sheds light on the anatomical logic of smell.Cell Rep. 2022 Mar 22;38(12):110547. doi: 10.1016/j.celrep.2022.110547. Cell Rep. 2022. PMID: 35320714 Free PMC article.
-
Is the mouse nose a miniature version of a rat nose? A computational comparative study.Comput Methods Programs Biomed. 2024 Sep;254:108282. doi: 10.1016/j.cmpb.2024.108282. Epub 2024 Jun 8. Comput Methods Programs Biomed. 2024. PMID: 38878359 Free PMC article.
References
-
- Bozza T., Vassalli A., Fuss S., Zhang J.-J., Weiland B., Pacifico R., Feinstein P. and Mombaerts P. (2009). Mapping of Class I and Class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron 61, 220-233. 10.1016/j.neuron.2008.11.010 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

