An uncharacterized region within the N-terminus of mouse TMC1 precludes trafficking to plasma membrane in a heterologous cell line
- PMID: 31649296
- PMCID: PMC6813322
- DOI: 10.1038/s41598-019-51336-0
An uncharacterized region within the N-terminus of mouse TMC1 precludes trafficking to plasma membrane in a heterologous cell line
Abstract
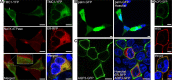
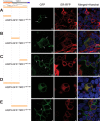
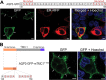
Mechanotransduction by hair cell stereocilia lies at the heart of sound detection in vertebrates. Considerable effort has been put forth to identify proteins that comprise the hair cell mechanotransduction apparatus. TMC1, a member of the transmembrane channel-like (TMC) family, was identified as a core protein of the mechanotransduction complex in hair cells. However, the inability of TMC1 to traffic through the endoplasmic reticulum in heterologous cellular systems has hindered efforts to characterize its function and fully identify its role in mechanotransduction. We developed a novel approach that allowed for the detection of uncharacterized protein regions, which preclude trafficking to the plasma membrane (PM) in heterologous cells. Tagging N-terminal fragments of TMC1 with Aquaporin 3 (AQP3) and GFP fusion reporter, which intrinsically label PM in HEK293 cells, indicated that residues at the edges of amino acid sequence 138-168 invoke intracellular localization and/or degradation. This signal is able to preclude surface localization of PM protein AQP3 in HEK293 cells. Substitutions of the residues by alanine or serine corroborated that the information determining the intracellular retention is present within amino acid sequence 138-168 of TMC1 N-terminus. This novel signal may preclude the proper trafficking of TMC1 to the PM in heterologous cells.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

