Cryo-Gel embedding compound for renal biopsy biobanking
- PMID: 31649317
- PMCID: PMC6813323
- DOI: 10.1038/s41598-019-51962-8
Cryo-Gel embedding compound for renal biopsy biobanking
Abstract
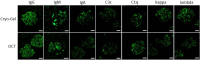
Optimal preservation and biobanking of renal tissue is vital for good diagnostics and subsequent research. Optimal cutting temperature (OCT) compound is a commonly used embedding medium for freezing tissue samples. However, due to interfering polymers in OCT, analysis as mass spectrometry (MS) is difficult. We investigated if the replacement of OCT with Cryo-Gel as embedding compound for renal biopsies would enable proteomics and not disturb other common techniques used in tissue diagnostics and research. For the present study, fresh renal samples were snap-frozen using Cryo-Gel, OCT and without embedding compound and evaluated using different techniques. In addition, tissue samples from normal spleen, skin, liver and colon were analyzed. Cryo-Gel embedded tissues showed good morphological preservation and no interference in immunohistochemical or immunofluorescent investigations. The quality of extracted RNA and DNA was good. The number of proteins identified using MS was similar between Cryo-Gel embedded samples, samples without embedding compound and OCT embedded samples. However, polymers in the OCT disturbed the signal in the MS, while this was not observed in the Cryo-Gel embedded samples. We conclude that embedding of renal biopsies in Cryo-Gel is an excellent and preferable alternative for OCT compound for both diagnostic and research purposes, especially in those cases where proteomic analysis might be necessary.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

