Region-Specific Response of Astrocytes to Prion Infection
- PMID: 31649496
- PMCID: PMC6794343
- DOI: 10.3389/fnins.2019.01048
Region-Specific Response of Astrocytes to Prion Infection
Abstract
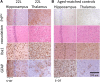
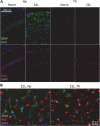
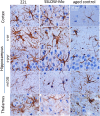
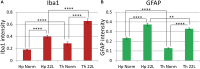
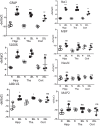
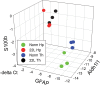
Chronic neuroinflammation involves reactive microgliosis and astrogliosis, and is regarded as a common pathological hallmark of neurodegenerative diseases including Alzheimer's, Parkinson's, ALS and prion diseases. Reactive astrogliosis, routinely observed immunohistochemically as an increase in glial fibrillary acidic protein (GFAP) signal, is a well-documented feature of chronic neuroinflammation associated with neurodegenerative diseases. Recent studies on single-cell transcriptional profiling of a mouse brain revealed that, under normal conditions, several distinct subtypes of astrocytes with regionally specialized distribution exist. However, it remains unclear whether astrocytic response to pro-inflammatory pathological conditions is uniform across whole brain or is region-specific. The current study compares the response of microglia and astrocytes to prions in mice infected with 22L mouse-adapted prion strain. While the intensity of reactive microgliosis correlated well with the extent of PrPSc deposition, reactive astrogliosis displayed a different, region-specific pattern. In particular, the thalamus and stratum oriens of hippocampus, which are both affected by 22L prions, displayed strikingly different response of astrocytes to PrPSc. Astrocytes in stratum oriens of hippocampus responded to accumulation of PrPSc with visible hypertrophy and increased GFAP, while in the thalamus, despite stronger PrPSc signal, the increase of GFAP was milder than in hippocampus, and the change in astrocyte morphology was less pronounced. The current study suggests that astrocyte response to prion infection is heterogeneous and, in part, defined by brain region. Moreover, the current work emphasizes the needs for elucidating region-specific changes in functional states of astrocytes and exploring the impact of these changes to chronic neurodegeneration.
Keywords: astrocytes; chronic neuroinflammation; hippocampus; microglia; prion; prion diseases; reactive astrogliosis; thalamus.
Copyright © 2019 Makarava, Chang, Kushwaha and Baskakov.
Figures





References
-
- Asuni A. A., Gray B., Bailey J., Skipp P., Perry V. H., O’Connor V. (2014). Analysis of the hippocampal proteome in ME7 prion disease reveals a predominant astrocytic signature and highlights the brain-restricted production of clusterin in chronic neurodegeneration. J. Biol. Chem. 289 4532–4545. 10.1074/jbc.M113.502690 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

