Microcins in Enterobacteriaceae: Peptide Antimicrobials in the Eco-Active Intestinal Chemosphere
- PMID: 31649628
- PMCID: PMC6795089
- DOI: 10.3389/fmicb.2019.02261
Microcins in Enterobacteriaceae: Peptide Antimicrobials in the Eco-Active Intestinal Chemosphere
Abstract
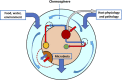
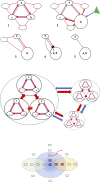
Microcins are low-molecular-weight, ribosomally produced, highly stable, bacterial-inhibitory molecules involved in competitive, and amensalistic interactions between Enterobacteriaceae in the intestine. These interactions take place in a highly complex chemical landscape, the intestinal eco-active chemosphere, composed of chemical substances that positively or negatively influence bacterial growth, including those originated from nutrient uptake, and those produced by the action of the human or animal host and the intestinal microbiome. The contribution of bacteria results from their effect on the host generated molecules, on food and digested food, and organic substances from microbial origin, including from bacterial degradation. Here, we comprehensively review the main chemical substances present in the human intestinal chemosphere, particularly of those having inhibitory effects on microorganisms. With this background, and focusing on Enterobacteriaceae, the most relevant human pathogens from the intestinal microbiota, the microcin's history and classification, mechanisms of action, and mechanisms involved in microcin's immunity (in microcin producers) and resistance (non-producers) are reviewed. Products from the chemosphere likely modulate the ecological effects of microcin activity. Several cross-resistance mechanisms are shared by microcins, colicins, bacteriophages, and some conventional antibiotics, which are expected to produce cross-effects. Double-microcin-producing strains (such as microcins MccM and MccH47) have been successfully used for decades in the control of pathogenic gut organisms. Microcins are associated with successful gut colonization, facilitating translocation and invasion, leading to bacteremia, and urinary tract infections. In fact, Escherichia coli strains from the more invasive phylogroups (e.g., B2) are frequently microcinogenic. A publicly accessible APD3 database http://aps.unmc.edu/AP/ shows particular genes encoding microcins in 34.1% of E. coli strains (mostly MccV, MccM, MccH47, and MccI47), and much less in Shigella and Salmonella (<2%). Some 4.65% of Klebsiella pneumoniae are microcinogenic (mostly with MccE492), and even less in Enterobacter or Citrobacter (mostly MccS). The high frequency and variety of microcins in some Enterobacteriaceae indicate key ecological functions, a notion supported by their dominance in the intestinal microbiota of biosynthetic gene clusters involved in the synthesis of post-translationally modified peptide microcins.
Keywords: Enterobacteriaceae; bacteriocins; chemosphere; colicins; competition; microcins; molecular ecology.
Copyright © 2019 Baquero, Lanza, Baquero, del Campo and Bravo-Vázquez.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

