Development of Potential Yeast Protein Extracts for Red Wine Clarification and Stabilization
- PMID: 31649649
- PMCID: PMC6794431
- DOI: 10.3389/fmicb.2019.02310
Development of Potential Yeast Protein Extracts for Red Wine Clarification and Stabilization
Abstract
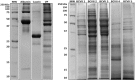
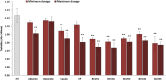
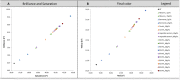
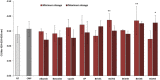
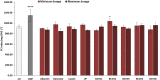
Recently, new technologies have been combined to improve quality and sensorial diversity of wine. Several fining agents were developed to induce flocculation and sedimentation of particulate matter in wine, enhancing its clarification, and stabilization. The fining agents most commonly used are animal proteins, such as milk casein or egg albumin. However, its use is being related to food intolerance. To overcome this issue, alternative sources should be explored for use in industrial processes. In previous studies performed by our consortium, the potential of yeast protein extracts (YPE) in white wine clarification, stabilization, and curative processes was identified. Thus, the main objective of the present work is to select YPE with the potential to develop fining agents for red wine, without health risk to consumers. Therefore, five yeast strains were selected from a diversified collection of oenological yeasts, in order to produce protein extracts. Along with the fining trials, a vinification assay was performed to evaluate the maceration effect of the obtained YPE. The previously selected yeast strains were also screened for the production of the usual enzymatic activities found in commercial maceration preparations, namely polygalacturonase, cellulase, protease, and ß-glucosidase activities, in order to evaluate its potential effect on wine. Our results indicate that YPE, particularly BCVII 1, BCVII 2, and BCVII 5 were able to promote a significant brilliance increase, along with a turbidity reduction and final color improvement. In the vinification assay, BCVII 2 stands out with better results for color intensity and phenolic compounds content improvement. In what refers to enzymatic activities, BCVII 2 shows advantage over the other YPEs, due to its protease and β-glucosidase activity. We demonstrate that the selected YPEs, with emphasis on BCVII 2, may represent an efficient alternative to the commonly used fining products.
Keywords: color; fining agents; turbidity; vinification; wine.
Copyright © 2019 Gaspar, Machado, Coutinho, Sousa, Santos, Xavier, Figueiredo, Teixeira, Centeno and Simões.
Figures


References
-
- Armada L., Falqué E. (2007). Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 225 553–558. 10.1007/s00217-006-0453-3 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

