Targeting the Janus Kinase Family in Autoimmune Skin Diseases
- PMID: 31649667
- PMCID: PMC6794457
- DOI: 10.3389/fimmu.2019.02342
Targeting the Janus Kinase Family in Autoimmune Skin Diseases
Abstract
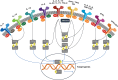
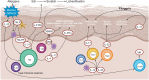
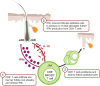
Autoimmune skin diseases are characterized by significant local and systemic inflammation that is largely mediated by the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway. Advanced understanding of this pathway has led to the development of targeted inhibitors of Janus kinases (JAKinibs). As a class, JAK inhibitors effectively treat a multitude of hematologic and inflammatory diseases. Growing evidence suggests that JAK inhibitors are efficacious in atopic dermatitis, alopecia areata, psoriasis, and vitiligo. Additional evidence suggests that JAK inhibition might be broadly useful in dermatology, with early reports of efficacy in several other conditions. JAK inhibitors can be administered orally or used topically and represent a promising new class of medications. Here we review the evolving data on the role of the JAK-STAT pathway in inflammatory dermatoses and the potential therapeutic benefit of JAK-STAT antagonism.
Keywords: Janus kinase; autoimmunity; cytokines; dermatology; skin barrier.
Copyright © 2019 Howell, Kuo and Smith.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

