Altering DNA Repair to Improve Radiation Therapy: Specific and Multiple Pathway Targeting
- PMID: 31649878
- PMCID: PMC6795692
- DOI: 10.3389/fonc.2019.01009
Altering DNA Repair to Improve Radiation Therapy: Specific and Multiple Pathway Targeting
Abstract
Radiation therapy (RT) is widely used in cancer care strategies. Its effectiveness relies mainly on its ability to cause lethal damage to the DNA of cancer cells. However, some cancers have shown to be particularly radioresistant partly because of efficient and redundant DNA repair capacities. Therefore, RT efficacy might be enhanced by using drugs that can disrupt cancer cells' DNA repair machinery. Here we review the recent advances in the development of novel inhibitors of DNA repair pathways in combination with RT. A large number of these compounds are the subject of preclinical/clinical studies and target key enzymes involved in one or more DNA repair pathways. A totally different strategy consists of mimicking DNA double-strand breaks via small interfering DNA (siDNA) to bait the whole DNA repair machinery, leading to its global inhibition.
Keywords: DNA damage; inhibition; radioresistance; radiotherapy; repair systems.
Copyright © 2019 Biau, Chautard, Verrelle and Dutreix.
Figures
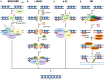
 Represents inhibitors of DNA repair in preclinical or clinical development.
Represents inhibitors of DNA repair in preclinical or clinical development.References
Publication types
LinkOut - more resources
Full Text Sources

