3D printing for airway disease
- PMID: 31650103
- PMCID: PMC6812574
- DOI: 10.21037/amj.2019.01.05
3D printing for airway disease
Abstract
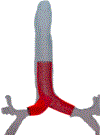
It has been 30 years since the first commercial three-dimensional (3D) printer was available on market. The technological advancement of 3D printing has far exceeded its implementation in medicine. The application of 3D printing technology has the potential of playing a major role within interventional pulmonology; specifically, in the management of complex airway disease. Tailoring management to the patient-specific anatomical malformation caused by benign or malignant disease is a major challenge faced by interventional pulmonologists. Such cases often require adjunctive therapeutic procedures with thermal therapies followed by dilation and airway stenting to maintain the patency of the airway. Airway-stent size matching is one key to reducing stent-related complications. A major barrier to matching is the expansion of the stent in two dimensions (fixed sizes in length and diameter) within the deformed airway. Additional challenges are created by the subjective oversizing of the stent to reduce the likelihood of migration. Improper sizing adversely affects the stability of the stent. The stent-airway mismatch can be complicated by airway erosion, perforation, or the formation of granulation tissue. Stents can migrate, fracture, obstruct, or become infected. The use of patient-specific 3D printed airway stents may be able to reduce the stent airway mismatch. These stents allow more precise stent-airway sizing and minimizes high-pressure points on distorted airway anatomy. In theory, this should reduce the incidence of the well-known complications of factory manufactured stents. In this article, the authors present the brief history of 3D printed stents, their consideration in select patients, processing steps for development, and future direction.
Keywords: Airway stent; bronchoscopy; three-dimensional printing (3D printing).
Conflict of interest statement
Conflicts of Interest: Cleveland Clinic and Cleveland Clinic Institutional Officials/Leaders have an equity interest in Custom Orthopaedic Solutions and are entitled to royalty payments from the company for technology developed at Cleveland Clinic. Custom Orthopaedic Solutions is the manufacturer the stent referrenced in the text. Dr. Gildea has Intellectual Property filed but no existing conflict as there is no commercial product at the time of this submission.
Figures






References
-
- Dutau H, Dumon JF. Airway Stenting Revisited: 30 Years, the Age of Reason? J Bronchology Interv Pulmonol 2017;24:257–9. - PubMed
-
- Antman EM, Loscalzo J. Precision medicine in cardiology. Nat Rev Cardiol 2016;13:591–602. - PubMed
-
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356–73. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
