Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL
- PMID: 31650176
- PMCID: PMC6933289
- DOI: 10.1182/blood.2019001641
Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL
Erratum in
-
Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134(26):2361-2368.Blood. 2020 Sep 10;136(11):1374. doi: 10.1182/blood.2020008394. Blood. 2020. PMID: 32957119 Free PMC article. No abstract available.
Abstract
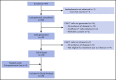
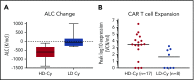
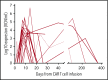
Chimeric antigen receptor (CAR) T cells have demonstrated clinical benefit in patients with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL). We undertook a multicenter clinical trial to determine toxicity, feasibility, and response for this therapy. A total of 25 pediatric/young adult patients (age, 1-22.5 years) with R/R B-ALL were treated with 19-28z CAR T cells. Conditioning chemotherapy included high-dose (3 g/m2) cyclophosphamide (HD-Cy) for 17 patients and low-dose (≤1.5 g/m2) cyclophosphamide (LD-Cy) for 8 patients. Fifteen patients had pretreatment minimal residual disease (MRD; <5% blasts in bone marrow), and 10 patients had pretreatment morphologic evidence of disease (≥5% blasts in bone marrow). All toxicities were reversible, including severe cytokine release syndrome in 16% (4 of 25) and severe neurotoxicity in 28% (7 of 25) of patients. Treated patients were assessed for response, and, among the evaluable patients (n = 24), response and peak CAR T-cell expansion were superior in the HD-Cy/MRD cohorts, as compared with the LD-Cy/morphologic cohorts without an increase in toxicity. Our data support the safety of CD19-specific CAR T-cell therapy for R/R B-ALL. Our data also suggest that dose intensity of conditioning chemotherapy and minimal pretreatment disease burden have a positive impact on response without a negative effect on toxicity. This trial was registered at www.clinicaltrials.gov as #NCT01860937.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: K.J.C. has received research support from Juno Therapeutics and Novartis and has consulted, served on advisory boards, and participated in educational seminars for Juno Therapeutics, Novartis, Gerson Lehrman Group (GLG), Geurson Medical Group, AXIS Education, and Omnicron Healthcare. S.P.M. has participated on advisory boards for Novartis. R.J.B., M.S., and I.R. are cofounders and receive royalties from Juno Therapeutics. C.S.S. has received research support from Juno Therapeutics and has consulted for or served on advisory boards of Juno Therapeutics, Kite, and Novartis. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Beat pediatric ALL MRD: CD28 CAR T and transplant.Blood. 2019 Dec 26;134(26):2333-2335. doi: 10.1182/blood.2019003821. Blood. 2019. PMID: 31877213 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. - PubMed
-
- Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373(16):1541-1552. - PubMed
-
- Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131(5):579-587. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

