The multi-site docking protein Grb2-associated binder 1 (Gab1) enhances interleukin-6-induced MAPK-pathway activation in an SHP2-, Grb2-, and time-dependent manner
- PMID: 31651330
- PMCID: PMC6814103
- DOI: 10.1186/s12964-019-0451-2
The multi-site docking protein Grb2-associated binder 1 (Gab1) enhances interleukin-6-induced MAPK-pathway activation in an SHP2-, Grb2-, and time-dependent manner
Abstract
Background: Cytokine-dependent activation of signalling pathways is tightly orchestrated. The spatiotemporal activation of signalling pathways dictates the specific physiological responses to cytokines. Dysregulated signalling accounts for neoplastic, developmental, and inflammatory diseases. Grb2-associated binder (Gab) family proteins are multi-site docking proteins, which expand cytokine-induced signal transduction in a spatial- and time-dependent manner by coordinating the recruitment of proteins involved in mitogen activated protein kinase (MAPK)/extracellular-signal regulated kinase (ERK) and phosphatidyl-inositol-3-kinase (PI3K) signalling. Interaction of Gab family proteins with these signalling proteins determines strength, duration and localization of active signalling cascades. However, the underlying molecular mechanisms of signal orchestration by Gab family proteins in IL-6-induced signalling are only scarcely understood.
Methods: We performed kinetic analyses of interleukin-6 (IL-6)-induced MAPK activation and analysed downstream responses. We compared signalling in wild-type cells, Gab1 knock-out cells, those reconstituted to express Gab1 mutants, and cells expressing gp130 receptors or receptor mutants.
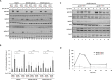
Results: Interleukin-6-induced MAPK pathway activation can be sub-divided into an early Gab1-independent and a subsequent Gab1-dependent phase. Early Gab1-independent MAPK activation is critical for the subsequent initiation of Gab1-dependent amplification of MAPK pathway activation and requires binding of SH2 domain-containing phosphatase 2 (SHP2) to the interleukin-6 receptor complex. Subsequent and coordinated recruitment of Grb2 and SHP2 to Gab1 is essential for Gab1-dependent amplification of IL-6-induced late MAPK pathway activation and subsequent gene expression.
Conclusions: Overall, we elaborated the molecular requirements for Gab1-dependent, spatiotemporal orchestration of interleukin-6-dependent MAPK signalling. We discriminated IL-6-induced Gab1-independent, early activation of MAPK signalling and Gab1-dependent, sustained activation of MAPK signalling.
Keywords: Cytokines; Erk; Gab1; IL-6; Interleukin-6; Jak; Janus kinase; MAPK; PI3K; SHP2; STAT; Signal orchestration; Signal transduction; c-Fos.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures










Similar articles
-
Signal strength dictates phosphoinositide 3-kinase contribution to Ras/extracellular signal-regulated kinase 1 and 2 activation via differential Gab1/Shp2 recruitment: consequences for resistance to epidermal growth factor receptor inhibition.Mol Cell Biol. 2008 Jan;28(2):587-600. doi: 10.1128/MCB.01318-07. Epub 2007 Nov 19. Mol Cell Biol. 2008. PMID: 18025104 Free PMC article.
-
A role for Grb2-associated binder-1 in growth hormone signaling.Endocrinology. 2002 Dec;143(12):4856-67. doi: 10.1210/en.2002-220565. Endocrinology. 2002. PMID: 12446613
-
Activation of the protein tyrosine phosphatase SHP2 via the interleukin-6 signal transducing receptor protein gp130 requires tyrosine kinase Jak1 and limits acute-phase protein expression.Biochem J. 1998 Nov 1;335 ( Pt 3)(Pt 3):557-65. doi: 10.1042/bj3350557. Biochem J. 1998. PMID: 9794795 Free PMC article.
-
The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway.Cell Signal. 2008 Mar;20(3):453-9. doi: 10.1016/j.cellsig.2007.10.002. Epub 2007 Oct 11. Cell Signal. 2008. PMID: 17993263 Review.
-
Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway.Biochem J. 1998 Sep 1;334 ( Pt 2)(Pt 2):297-314. doi: 10.1042/bj3340297. Biochem J. 1998. PMID: 9716487 Free PMC article. Review.
Cited by
-
Grb2 Induces Cardiorenal Syndrome Type 3: Roles of IL-6, Cardiomyocyte Bioenergetics, and Akt/mTOR Pathway.Front Cell Dev Biol. 2021 Mar 22;9:630412. doi: 10.3389/fcell.2021.630412. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33829014 Free PMC article.
-
IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer.Biomed Res Int. 2021 Apr 23;2021:5535578. doi: 10.1155/2021/5535578. eCollection 2021. Biomed Res Int. 2021. PMID: 33981768 Free PMC article.
-
The tyrosine phosphatase SHP2 increases robustness and information transfer within IL-6-induced JAK/STAT signalling.Cell Commun Signal. 2021 Sep 16;19(1):94. doi: 10.1186/s12964-021-00770-7. Cell Commun Signal. 2021. PMID: 34530865 Free PMC article.
-
The Role of GAB1 in Cancer.Cancers (Basel). 2023 Aug 20;15(16):4179. doi: 10.3390/cancers15164179. Cancers (Basel). 2023. PMID: 37627207 Free PMC article. Review.
-
Comparison between Heat-Clearing Medicine and Antirheumatic Medicine in Treatment of Gastric Cancer Based on Network Pharmacology, Molecular Docking, and Tumor Immune Infiltration Analysis.Evid Based Complement Alternat Med. 2022 Jan 11;2022:7490279. doi: 10.1155/2022/7490279. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35069767 Free PMC article.
References
-
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. - PubMed
-
- Schiemann WP, Bartoe JL, Nathanson NM. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. Evidence for participation of multiple signaling pathways which converge at Ras. J Biol Chem. 1997;272:16631–16636. doi: 10.1074/jbc.272.26.16631. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

