Treatment of Symptomatic Convergence Insufficiency in Children Enrolled in the Convergence Insufficiency Treatment Trial-Attention & Reading Trial: A Randomized Clinical Trial
- PMID: 31651593
- PMCID: PMC6855327
- DOI: 10.1097/OPX.0000000000001443
Treatment of Symptomatic Convergence Insufficiency in Children Enrolled in the Convergence Insufficiency Treatment Trial-Attention & Reading Trial: A Randomized Clinical Trial
Abstract
Significance: These data confirm the effectiveness of office-based vergence/accommodative therapy for improving convergence in children with symptomatic convergence insufficiency. They also highlight the importance of using a primary outcome measure that is as objective as possible rather than relying solely on self-reported symptoms for studies of binocular vision in children.
Purpose: The purpose of this study was to report changes in clinical signs and symptoms of convergence insufficiency (secondary outcome measures) from a multicenter clinical trial (Convergence Insufficiency Treatment Trial-Attention & Reading Trial [CITT-ART]) evaluating the effectiveness of vergence/accommodative therapy for improving reading and attention in children with symptomatic convergence insufficiency.
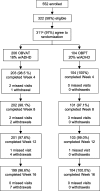
Methods: Three hundred eleven children aged 9 to 14 years with symptomatic convergence insufficiency were randomly assigned to 16 weeks of office-based vergence/accommodative therapy or to placebo therapy. Improvements in (1) near point of convergence (NPC), (2) positive fusional vergence (PFV), and (3) self-reported symptoms (Convergence Insufficiency Symptom Survey [CISS] score) were compared after 16 weeks of treatment.
Results: Mean NPC improved 10.4 cm in the vergence/accommodative and 6.2 cm in the placebo therapy group (mean difference of -4.2 cm [95% confidence interval {CI}, -5.2 to -3.2 cm; P < .001]); mean PFV increased 23.2 and 8.8Δ in the vergence/accommodative and placebo therapy groups, respectively (mean difference of 14.4Δ [95% CI, 12.1 to 16.8Δ; P < .001]). The mean CISS score improved 11.8 and 10.4 points in the vergence/accommodative and placebo therapy groups, respectively (mean difference of 1.5 points [95% CI, -3.8 to +0.8 points; P = .21]).
Conclusions: Our results demonstrate that office-based vergence/accommodative therapy is effective for improving the NPC and PFV in children with symptomatic convergence insufficiency. However, given that both treatment groups had a similar reduction in self-reported symptoms, it may not be prudent to use the CISS alone as a measure of successful treatment.
Trial registration: ClinicalTrials.gov NCT00338611.
Conflict of interest statement
Figures
References
-
- Rouse MW, Borsting E, Hyman L, et al. Frequency of Convergence Insufficiency among Fifth and Sixth Graders. The Convergence Insufficiency and Reading Study (CIRS) Group. Optom Vis Sci 1999;76:643–9. - PubMed
-
- Scheiman M, Mitchell GL, Cotter S, et al. A Randomized Trial of the Effectiveness of Treatments for Convergence Insufficiency in Children. Arch Ophthalmol 2005;123:14–24. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical