Jasmonate Signaling during Arabidopsis Stamen Maturation
- PMID: 31651948
- PMCID: PMC6896695
- DOI: 10.1093/pcp/pcz201
Jasmonate Signaling during Arabidopsis Stamen Maturation
Abstract
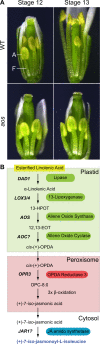
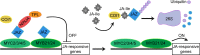
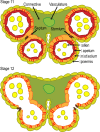
The last stages of stamen development, collectively called stamen maturation, encompass pollen viability, filament elongation and anther dehiscence or opening. These processes are essential for male fertility in Arabidopsis and require the function of jasmonate signaling. There is a good understanding of jasmonate synthesis, perception and transcriptional outputs in Arabidopsis stamens. In addition, the spatiotemporal localization of jasmonate signaling components at the tissue and cellular levels has started to emerge in recent years. However, the ultimate cellular functions activated by jasmonate to promote stamen maturation remain unknown. The hormones auxin and gibberellin have been proposed to control the activation of jasmonate synthesis to promote stamen maturation, although we hypothesize that this action is rather indirect. In this review, we examine these different areas, attempt to clarify some confusing aspects found in the literature and raise testable hypothesis that may help to further understand how jasmonate controls male fertility in Arabidopsis.
Keywords: Anther dehiscence; Auxin; Filament elongation; Jasmonate; Pollen viability; Stamen maturation.
� The Author(s) 2019. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Figures

Comment in
-
Letter to the Editor: Author Response-The Role of Auxin in Late Stamen Development.Plant Cell Physiol. 2020 Sep 1;61(9):1533-1534. doi: 10.1093/pcp/pcaa088. Plant Cell Physiol. 2020. PMID: 32592487 Free PMC article. No abstract available.
-
Letter to the Editor: The Role of Auxin in Late Stamen Development.Plant Cell Physiol. 2020 Sep 1;61(9):1531-1532. doi: 10.1093/pcp/pcaa089. Plant Cell Physiol. 2020. PMID: 32592488 No abstract available.
References
-
- Banerjee S., Bose I. (2011) Transient pulse formation in jasmonate signaling pathway. J. Theor. Biol. 273: 188–196. - PubMed
-
- Bannenberg G., Martinez M., Hamberg M., Castresana C. (2009) Diversity of the enzymatic activity in the lipoxygenase gene family of Arabidopsis thaliana. Lipids 44: 85–95. - PubMed
-
- Bonner L.J., Dickinson H.G. (1990) Anther dehiscence in Lycopersicon esculentum. 2. Water relations. New Phytol. 115: 367–375. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

