Proficiency Testing of Viral Marker Screening in African Blood Centers - Seven African Countries, 2017
- PMID: 31652252
- PMCID: PMC6812837
- DOI: 10.15585/mmwr.mm6842a3
Proficiency Testing of Viral Marker Screening in African Blood Centers - Seven African Countries, 2017
Abstract
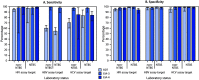
A 2014 report evaluating accuracy of serologic testing for transfusion-transmissible viruses at African blood center laboratories found sensitivities of 92%, 87%, and 90% for detecting infections with human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), respectively (1). Following substantial investments in national blood transfusion service (NBTS) laboratories, in 2017 investigators tested proficiency at 84 blood center laboratories (29 NBTS and 55 non-NBTS) in seven African countries. A blinded panel of 25 plasma samples was shipped to each participating laboratory for testing with their usual protocols based on rapid diagnostic tests (RDTs) (2) and third and fourth generation enzyme immunoassays (EIA-3 and EIA-4). Sensitivity and specificity were estimated using separate regression models that clustered assays by laboratory and adjusted for assay type and NBTS laboratory status. Mean specificities were ≥95% for all three viruses; however, mean sensitivities were 97% for HIV-positive, 76% for HBV-positive, and 80% for HCV-positive samples. Testing sensitivities for all viruses were high when EIA-3 assays were used (≥97%). Lower sensitivities for HBV-positive samples and HCV-positive samples were associated with assay types other than EIA-3, used primarily by non-NBTS laboratories. Proficiency for HIV testing has improved following international investments, but proficiency remains suboptimal for HBV and HCV testing. In sub-Saharan African blood centers, the quality of rapid tests used for HBV and HCV screening needs to be improved or their use discouraged in favor of EIA-3 tests.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures