Adjusted treatment COMPArisons between guSelkumab and uStekinumab for treatment of moderate-to-severe plaque psoriasis: the COMPASS analysis
- PMID: 31652347
- PMCID: PMC7496582
- DOI: 10.1111/bjd.18634
Adjusted treatment COMPArisons between guSelkumab and uStekinumab for treatment of moderate-to-severe plaque psoriasis: the COMPASS analysis
Abstract
Background: Guselkumab is an interleukin-23 inhibitor indicated for the treatment of moderate-to-severe plaque psoriasis in adults. Guselkumab has demonstrated additional benefit in patients with early inadequate response to ustekinumab. Long-term efficacy comparisons of guselkumab and ustekinumab are currently lacking among ustekinumab-naive patients.
Objectives: To assess the relative efficacy of guselkumab and ustekinumab for maintenance therapy of moderate-to-severe plaque psoriasis, using individual patient data (IPD) from randomized controlled trials.
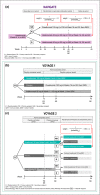
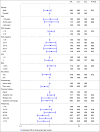
Methods: IPD for guselkumab from the VOYAGE 1 and 2 trials were pooled and compared with IPD for ustekinumab from the NAVIGATE trial. Multivariable logistic regression analyses compared guselkumab 100 mg and ustekinumab 45 mg or 90 mg for the achievement and maintenance of Psoriasis Area and Severity Index (PASI) 90, 75 and 100 responses up to 40 weeks. The regression models accounted for a range of clinically relevant covariates (e.g. age, sex, psoriasis duration). Relative efficacy was expressed using odds ratios (ORs) and predicted probability of treatment response associated with each intervention.
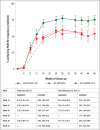
Results: Patients receiving guselkumab had significantly higher probabilities of achieving a PASI 90 response than patients receiving ustekinumab, at both week 16 [70·4% vs. 46·0%, OR 2·79, 95% confidence interval (CI) 2·22-3·45] and week 40 (74·2% vs. 54·5%, OR 2·40, 95% CI 1·89-3·13]. Guselkumab was also associated with a significantly increased likelihood of achieving both PASI 75 and PASI 100 responses at weeks 16 and 40, compared with ustekinumab.
Conclusions: Adjusted analyses leveraging IPD demonstrate that guselkumab has a significantly higher probability of achieving and maintaining PASI treatment responses through week 40 than ustekinumab does.
© 2019 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures


Comment in
-
Repurposing existing trial data to infer relative efficacy of biologics: guselkumab vs. ustekinumab for psoriasis.Br J Dermatol. 2020 Aug;183(2):202-203. doi: 10.1111/bjd.18848. Epub 2020 Jan 14. Br J Dermatol. 2020. PMID: 31943131 No abstract available.
References
-
- Queiro R, Tejon P, Alonso S et al Age at disease onset: a key factor for understanding psoriatic disease. Rheumatology (Oxford) 2014; 53:1178–85. - PubMed
-
- World Health Organization . Global Report on Psoriasis. World Health Organization, 2016. Available at: https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_en... (last accessed 8 November 2019).
-
- Lebwohl MG, Bachelez H, Barker J et al Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014; 70:871–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

