Screening a Broad Range of Solid and Haematological Tumour Types for CD70 Expression Using a Uniform IHC Methodology as Potential Patient Stratification Method
- PMID: 31652572
- PMCID: PMC6826714
- DOI: 10.3390/cancers11101611
Screening a Broad Range of Solid and Haematological Tumour Types for CD70 Expression Using a Uniform IHC Methodology as Potential Patient Stratification Method
Abstract
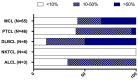
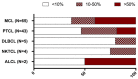
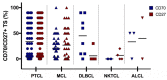
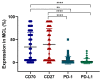
The constitutive expression of CD70 has been described in various haematological and solid tumour types. In addition, the co-expression of its receptor in tumours has been demonstrated, mediating tumour cell proliferation. Although CD70 expression is a prerequisite to enrol patients in solid tumour clinical trials using anti-CD70 immunotherapy, there is currently no standardised test to evaluate CD70 expression. These differences in immunohistochemistry (IHC) protocols make it challenging to compare the expression levels that were obtained in different studies, pointing out the need for one uniform methodology. In this retrospective study, over 600 tumour samples from different solid and haematological malignancies were analysed while using one validated IHC method. CD70 and CD27 expression was demonstrated in a broad range of tumour types. In solid tumours, 43% demonstrated CD70 positivity with the highest degree in renal cell carcinoma (79.5%). Kaposi sarcoma showed no CD70 expression on the tumour cells. In lymphoma samples, 58% demonstrated CD70 positivity. Moreover, the co-expression of CD70 and CD27 was observed in 39% of lymphoma samples. These findings highlight the need to further explore anti-CD70 therapies in a broad range of CD70 expressing tumour types and in doing so, implementing one standardised protocol to define CD70 overexpression to use it as a diagnostic tool.
Keywords: CD27; CD70; biomarker; immune checkpoint; immunohistochemistry.
Conflict of interest statement
The authors declare no conflict of interest. L.V.R., H.d.H., hold ownership interest (including patents) in argenx. V.R. is a consultant/advisory board member for Infinity Pharmaceuticals, Bristol-Myers Squibb, Eisai, PharmaMar, Gilead Sciences, NanoString Technologies, Incyte, BMS, MSD, Roche/Genentech, Epizyme, Astra Zeneca and Servier.
Figures




References
-
- Nolte M.A., van Olffen R.W., van Gisbergen K.P.J.M., van Lier R.A.W. Timing and tuning of CD27-CD70 interactions: The impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol. Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

