Ivalin Induces Mitochondria-Mediated Apoptosis Associated with the NF-κB Activation in Human Hepatocellular Carcinoma SMMC-7721 Cells
- PMID: 31652659
- PMCID: PMC6832439
- DOI: 10.3390/molecules24203809
Ivalin Induces Mitochondria-Mediated Apoptosis Associated with the NF-κB Activation in Human Hepatocellular Carcinoma SMMC-7721 Cells
Abstract
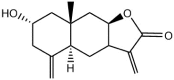
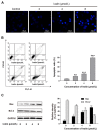
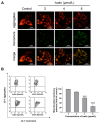
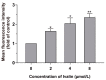
Ivalin, a natural compound isolated from Carpesium divaricatum, showed excellent microtubule depolymerization activities among human hepatocellular carcinoma in our previous work. Here, we investigated its functions on mitochondria-mediated apoptosis in hepatocellular carcinoma SMMC-7721 cells. DAPI (4',6-diamidino-2-phenylindole) staining, annexin V-fluorexcein isothiocyanate (FITC) apoptosis detection, and western blotting were applied to explore the apoptotic effect of Ivalin. Next, the induction effect of Ivalin on the mitochondrial pathway was also confirmed via a series of phenomena including the damage of mitochondria membrane potential, mitochondria cytochrome c escape, cleaved caspase-3 induction, and the reactive oxygen species generation. In this connection, we understood that Ivalin induced apoptosis through the mitochondrial pathway and the overload of reactive oxygen species. Furthermore, we found that the activation of nuclear factor-κB (NF-κB) and subsequent p53 induction were associated with the apoptotic effect of Ivalin. These data confirmed that Ivalin might be a promising pro-apoptotic compound that can be utilized as a potential drug for clinical treatment.
Keywords: Carpesium divaricatum; Ivalin; NF-κB; hepatocellular carcinoma; mitochondria-mediated apoptosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

