Metabolic Cytokines at Fasting and During Macronutrient Challenges: Influence of Obesity, Female Androgen Excess and Sex
- PMID: 31652917
- PMCID: PMC6893420
- DOI: 10.3390/nu11112566
Metabolic Cytokines at Fasting and During Macronutrient Challenges: Influence of Obesity, Female Androgen Excess and Sex
Abstract
Scope: Cytokines have pleiotropic functions within the organism and their levels may be influenced by obesity, visceral adiposity and sex hormones. Diet composition may also affect their systemic concentrations during fasting and in the postprandial period. Hence, we studied the influence of sex steroids and obesity on the circulating levels of a panel of metabolic cytokines in the fasting state and after single macronutrient challenges.
Methods: On alternate days we submitted 17 women with polycystic ovary syndrome (PCOS) (9 non-obese, 8 obese), 17 non-hyperandrogenic control women (9 non-obese, 8 obese) and 19 control men (10 non-obese, 9 obese) to isocaloric oral glucose, lipid and protein loads. Serum levels of omentin-1, vaspin, lipocalin-2, adipsin, PAI-1, chemerin, FGF-21 and FGF-23 were determined by Luminex multiplex technology.
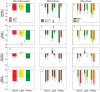
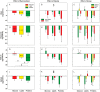
Results: During fasting, obese patients presented higher levels of PAI-1, chemerin and adipsin but decreased FGF-23 and omentin-1 compared with non-obese subjects. Vaspin showed sexual dimorphism with lower levels in men than women with PCOS and female controls. Following macronutrient ingestion, most metabolic cytokines presented a similar physiological response consisting of a decrease in circulating concentrations, which was inversely associated with the fasting levels of these molecules. Protein intake caused the major postprandial decrease whereas glucose did not significantly reduce PAI-1, FGF-23 and vaspin, and even increased FGF-21. Regardless of the macronutrient administered, vaspin levels showed a larger reduction in non-obese individuals while the decrease in PAI-1 was particularly noticeable in the obese subgroup. The postprandial reductions of omentin-1 and FGF-23 after glucose and protein loads were influenced by obesity. No major differences were found between patients with PCOS and male and female controls.
Conclusions: Obesity, but not PCOS or sex, markedly influences metabolic cytokine levels at fasting and after macronutrient ingestion. The observed postprandial decrease in their circulating concentrations might represent a physiological compensatory mechanism against food-induced inflammation and oxidative stress. This mechanism is altered by obesity and is differently modulated by macronutrients, suggesting a larger contribution of glucose to stressful postprandial responses.
Keywords: PCOS; adipokines; adiposity; glucose; lipids; oral loads; proteins; sex hormones.
Conflict of interest statement
The Authors have no conflict of Interest to disclose.
Figures
References
-
- Petersen P.S., Lei X., Seldin M.M., Rodriguez S., Byerly M.S., Wolfe A., Whitlock S., Wong G.W. Dynamic and extensive metabolic state-dependent regulation of cytokine expression and circulating levels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R1458–R1470. doi: 10.1152/ajpregu.00335.2014. - DOI - PMC - PubMed
-
- Luque-Ramirez M., Martinez-Garcia M.A., Montes-Nieto R., Fernandez-Duran E., Insenser M., Alpanes M., Escobar-Morreale H.F. Sexual dimorphism in adipose tissue function as evidenced by circulating adipokine concentrations in the fasting state and after an oral glucose challenge. Hum. Reprod. 2013;28:1908–1918. doi: 10.1093/humrep/det097. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

