Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy-Review of the Literature and Future Outlook
- PMID: 31653079
- PMCID: PMC6912719
- DOI: 10.3390/jcm8111777
Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy-Review of the Literature and Future Outlook
Abstract
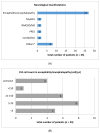
Immune checkpoint inhibitor (ICI) therapy has revolutionized the management of various cancers with previously poor prognosis. Despite its great efficacy, the therapy is associated with a wide spectrum of immune-related adverse events (irAE) including neurological symptoms which can affect all parts of the central and peripheral nervous system. Even though these events are rare, they are of high relevance as the rate of residual symptoms or even fatal outcomes is remarkable. To provide a detailed overview of neurological adverse events associated with immune checkpoint-inhibitor therapy we conducted a literature search. While focusing on ipilimumab, nivolumab, and pembrolizumab therapy, all available case reports as well as larger case series and clinical trials have been considered. Eighty-two case reports about checkpoint-inhibitor therapy induced symptoms of the peripheral nervous system have been published, while only 43 case reports addressed central nervous system abnormalities. The frequency of immune checkpoint-inhibitor therapy inducing neurological adverse events is about 1% in larger studies. Especially neuromuscular adverse events exhibit distinct clinical and diagnostic characteristics. Additionally, several affected patients presented with overlap-syndromes, which means that symptoms and diagnostic findings indicating myositis, myasthenia gravis, and neuropathy were present in one individual patient at the same time. Thus, neurological and particularly neuromuscular adverse events of immune checkpoint-inhibitor therapy may constitute a new disease entity.
Keywords: Guillain-Barré syndrome; encephalitis; immune checkpoint inhibitor therapy; immune-related adverse events; ipilimumab; myasthenia gravis; myositis; nivolumab; pembrolizumab.
Conflict of interest statement
N.M. declares no conflict of interest. G.B. received research support from the Bundesministerium für Bildung und Forschung (BMBF) and Deutsche Gesellschaft für internistitische Intensiv- und Notfallmedizin (DGIIN). He received travel support/honoraria from Deutsche Gesellschaft für internistitische Intensiv- und Notfallmedizin (DGIIN), Deutsche Gesellschaft für Hämatologie und Onkologie (DGHO), Jazz Pharmaceuticals, MSD, and NewConceptOncology. R.G. received research support (to institution) from Pfizer, Johnson&Johnson, Novartis, Amgen, and MerckSerono. He got honoraria (to person) from RochePharma, Bristol-Myers-Squibb, Novartis, Merck MSD, Almirall-Hermal, Amgen, Merck-Serono, SUN, Pierre-Fabre, Sanofi, and 4SC. P.I. received research support from BMS, Bayer, Calithera, Lilly, Merck, Novartis, EISAI, Pfizer, MSD, AstraZeneca, Roche, and Ipsen. He got lecture honoraria from AIM, AstraZeneca, BMS, Bayer, DKG-Onko, EISAI, EUSA, Ipsen, Merck, MedKom, MedWiss, Novartis, Pfizer, Roche, and StreamedUP! He had an advisory role for BMS, Bayer, Ipsen, Merck, Novartis, Pfizer, and Roche. I.S. received honoraria from BMS, Novartis, Roche, and Sanofi. T.S. received honoraria from Alexion, Bayer Vital GmbH, CSL Behring, Merck, Novartis, and Sanofi.
Figures


References
-
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

