Simultaneous Release of Multiple Vesicles from Rods Involves Synaptic Ribbons and Syntaxin 3B
- PMID: 31653448
- PMCID: PMC7036726
- DOI: 10.1016/j.bpj.2019.10.006
Simultaneous Release of Multiple Vesicles from Rods Involves Synaptic Ribbons and Syntaxin 3B
Abstract
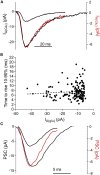
First proposed as a specialized mode of release at sensory neurons possessing ribbon synapses, multivesicular release has since been described throughout the central nervous system. Many aspects of multivesicular release remain poorly understood. We explored mechanisms underlying simultaneous multivesicular release at ribbon synapses in salamander retinal rod photoreceptors. We assessed spontaneous release presynaptically by recording glutamate transporter anion currents (IA(glu)) in rods. Spontaneous IA(glu) events were correlated in amplitude and kinetics with simultaneously measured miniature excitatory postsynaptic currents in horizontal cells. Both measures indicated that a significant fraction of events is multiquantal, with an analysis of IA(glu) revealing that multivesicular release constitutes ∼30% of spontaneous release events. IA(glu) charge transfer increased linearly with event amplitude showing that larger events involve greater glutamate release. The kinetics of large and small IA(glu) events were identical as were rise times of large and small miniature excitatory postsynaptic currents, indicating that the release of multiple vesicles during large events is highly synchronized. Effects of exogenous Ca2+ buffers suggested that multiquantal, but not uniquantal, release occurs preferentially near Ca2+ channels clustered beneath synaptic ribbons. Photoinactivation of ribbons reduced the frequency of spontaneous multiquantal events without affecting uniquantal release frequency, showing that spontaneous multiquantal release requires functional ribbons. Although both occur at ribbon-style active zones, the absence of cross-depletion indicates that evoked and spontaneous multiquantal release from ribbons involve different vesicle pools. Introducing an inhibitory peptide into rods to interfere with the SNARE protein, syntaxin 3B, selectively reduced multiquantal event frequency. These results support the hypothesis that simultaneous multiquantal release from rods arises from homotypic fusion among neighboring vesicles on ribbons and involves syntaxin 3B.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

