Insights into lipid accumulation in skeletal muscle in dysferlin-deficient mice
- PMID: 31653658
- PMCID: PMC6889719
- DOI: 10.1194/jlr.RA119000399
Insights into lipid accumulation in skeletal muscle in dysferlin-deficient mice
Abstract
Loss of dysferlin (DYSF) protein in humans results in limb-girdle muscular dystrophy 2B, characterized by progressive loss of muscles in the distal limbs with impaired locomotion. The DYSF-null (Bla/J) mouse develops severe steatotic muscles upon aging. Here, we report a marked increase in adipocytes, especially in the psoas and gluteus muscles but not in the soleus and tibialis anterior muscles in aged Bla/J mice compared with WT mice. There was a robust upregulation in the mRNA expression of enzymes involved in lipogenesis and triacylglycerol (TAG) synthesis pathways in the steatotic skeletal muscles. Lipidomic analysis of the steatotic skeletal muscles revealed an increase in several molecular species of TAG, although it is unclear whether it was at the expense of phosphatidylcholine and phosphatidylserine. The adipocytes in steatotic muscles were extramyocellular, as determined by the increased expression of caveolin 1 (a cellular marker for adipocytes) and lipid-droplet protein, perilipin 1. This increase in adipocytes occured as a consequence of the loss of myocytes.
Keywords: Bla/J mice; adipose tissue; extra-myocellular adipocytes; limb-girdle muscular dystrophy; lipidomic.
Copyright © 2019 Agarwal et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
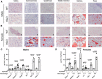
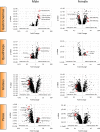





References
-
- Taghizadeh E., Rezaee M., Barreto G. E., and Sahebkar A.. 2019. Prevalence, pathological mechanisms, and genetic basis of limb-girdle muscular dystrophies: a review. J. Cell. Physiol. 234: 7874–7884. - PubMed
-
- Liu J., Aoki M., Illa I., Wu C., Fardeau M., Angelini C., Serrano C., Urtizberea J. A., Hentati F., Hamida M. B., et al. 1998. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat. Genet. 20: 31–36. - PubMed
-
- Amato A. A., and Brown R. H. Jr.. 2011. Dysferlinopathies. Handb. Clin. Neurol. 101: 111–118. - PubMed
-
- Moore U., Jacobs M., James M. K., Mayhew A. G., Fernandez-Torron R., Feng J., Cnaan A., Eagle M., Bettinson K., Rufibach L. E., et al. ; Jain COS Consortium. Assessment of disease progression in dysferlinopathy: a 1-year cohort study. Neurology. Epub ahead of print. January 9, 2019; doi:10.1212/WNL.0000000000006858. - PMC - PubMed
-
- Grounds M. D., Terrill J. R., Radley-Crabb H. G., Robertson T., Papadimitriou J., Spuler S., and Shavlakadze T.. 2014. Lipid accumulation in dysferlin-deficient muscles. Am. J. Pathol. 184: 1668–1676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

