Impaired tau-microtubule interactions are prevalent among pathogenic tau variants arising from missense mutations
- PMID: 31653695
- PMCID: PMC6885647
- DOI: 10.1074/jbc.RA119.010178
Impaired tau-microtubule interactions are prevalent among pathogenic tau variants arising from missense mutations
Abstract
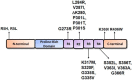
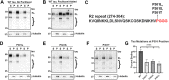
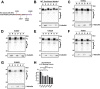
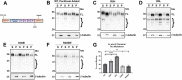
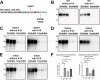
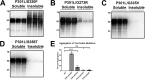
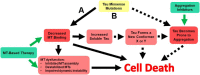
tau is a microtubule (MT)-associated protein that promotes tubulin assembly and stabilizes MTs by binding longitudinally along the MT surface. tau can aberrantly aggregate into pathological inclusions that define Alzheimer's disease, frontotemporal dementias, and other tauopathies. A spectrum of missense mutations in the tau-encoding gene microtubule-associated protein tau (MAPT) can cause frontotemporal dementias. tau aggregation is postulated to spread by a prion-like mechanism. Using a cell-based inclusion seeding assay, we recently reported that only a few tau variants are intrinsically prone to this type of aggregation. Here, we extended these studies to additional tau mutants and investigated their MT binding properties in mammalian cell-based assays. A limited number of tau variants exhibited modest aggregation propensity in vivo, but most tau mutants did not aggregate. Reduced MT binding appeared to be the most common dysfunction for the majority of tau variants due to missense mutations, implying that MT-targeting therapies could potentially be effective in the management of tauopathies.
Keywords: Alzheimer disease; aggregation; microtubule; prion; tau protein (tau); tauopathy.
© 2019 Xia et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Jack C. R. Jr., Bennett D. A., Blennow K., Carrillo M. C., Dunn B., Haeberlein S. B., Holtzman D. M., Jagust W., Jessen F., Karlawish J., Liu E., Molinuevo J. L., Montine T., Phelps C., Rankin K. P., et al. (2018) NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 14, 535–562 10.1016/j.jalz.2018.02.018 - DOI - PMC - PubMed
-
- Nelson P. T., Alafuzoff I., Bigio E. H., Bouras C., Braak H., Cairns N. J., Castellani R. J., Crain B. J., Davies P., Del Tredici K., Duyckaerts C., Frosch M. P., Haroutunian V., Hof P. R., Hulette C. M., et al. (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 71, 362–381 10.1097/NEN.0b013e31825018f7 - DOI - PMC - PubMed
-
- Nelson P. T., Jicha G. A., Schmitt F. A., Liu H., Davis D. G., Mendiondo M. S., Abner E. L., and Markesbery W. R. (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J. Neuropathol. Exp. Neurol. 66, 1136–1146 10.1097/nen.0b013e31815c5efb - DOI - PMC - PubMed
-
- Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., et al. (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 10.1038/31508 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

