Comparative Genomics of Rumen Butyrivibrio spp. Uncovers a Continuum of Polysaccharide-Degrading Capabilities
- PMID: 31653790
- PMCID: PMC6912079
- DOI: 10.1128/AEM.01993-19
Comparative Genomics of Rumen Butyrivibrio spp. Uncovers a Continuum of Polysaccharide-Degrading Capabilities
Abstract
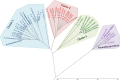
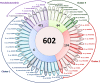
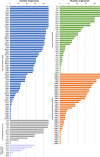
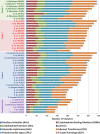
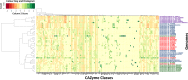
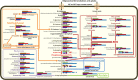
Plant polysaccharide breakdown by microbes in the rumen is fundamental to digestion in ruminant livestock. Bacterial species belonging to the rumen genera Butyrivibrio and Pseudobutyrivibrio are important degraders and utilizers of lignocellulosic plant material. These bacteria degrade polysaccharides and ferment the released monosaccharides to yield short-chain fatty acids that are used by the ruminant for growth and the production of meat, milk, and fiber products. Although rumen Butyrivibrio and Pseudobutyrivibrio species are regarded as common rumen inhabitants, their polysaccharide-degrading and carbohydrate-utilizing enzymes are not well understood. In this study, we analyzed the genomes of 40 Butyrivibrio and 6 Pseudobutyrivibrio strains isolated from the plant-adherent fraction of New Zealand dairy cows to explore the polysaccharide-degrading potential of these important rumen bacteria. Comparative genome analyses combined with phylogenetic analysis of their 16S rRNA genes and short-chain fatty acid production patterns provide insight into the genomic diversity and physiology of these bacteria and divide Butyrivibrio into 3 species clusters. Rumen Butyrivibrio bacteria were found to encode a large and diverse spectrum of degradative carbohydrate-active enzymes (CAZymes) and binding proteins. In total, 4,421 glycoside hydrolases (GHs), 1,283 carbohydrate esterases (CEs), 110 polysaccharide lyases (PLs), 3,605 glycosyltransferases (GTs), and 1,706 carbohydrate-binding protein modules (CBM) with predicted activities involved in the depolymerization and transport of the insoluble plant polysaccharides were identified. Butyrivibrio genomes had similar patterns of CAZyme families but varied greatly in the number of genes within each category in the Carbohydrate-Active Enzymes database (CAZy), suggesting some level of functional redundancy. These results suggest that rumen Butyrivibrio species occupy similar niches but apply different degradation strategies to be able to coexist in the rumen.IMPORTANCE Feeding a global population of 8 billion people and climate change are the primary challenges facing agriculture today. Ruminant livestock are important food-producing animals, and maximizing their productivity requires an understanding of their digestive systems and the roles played by rumen microbes in plant polysaccharide degradation. Members of the genera Butyrivibrio and Pseudobutyrivibrio are a phylogenetically diverse group of bacteria and are commonly found in the rumen, where they are a substantial source of polysaccharide-degrading enzymes for the depolymerization of lignocellulosic material. Our findings have highlighted the immense enzymatic machinery of Butyrivibrio and Pseudobutyrivibrio species for the degradation of plant fiber, suggesting that these bacteria occupy similar niches but apply different degradation strategies in order to coexist in the competitive rumen environment.
Keywords: Butyrivibrio; CAZy; Pseudobutyrivibrio; bacteria; enolase; genome; polysaccharide; rumen.
Copyright © 2019 Palevich et al.
Figures
References
-
- Hess M, Sczyrba A, Egan R, Kim T-W, Chokhawala H, Schroth G, Luo S, Clark DS, Chen F, Zhang T, Mackie RI, Pennacchio LA, Tringe SG, Visel A, Woyke T, Wang Z, Rubin EM. 2011. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 331:463–467. doi:10.1126/science.1200387. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

