Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions
- PMID: 31653845
- PMCID: PMC6814866
- DOI: 10.1038/s41467-019-12773-7
Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions
Abstract
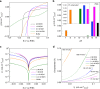
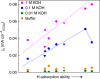
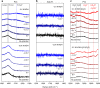
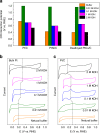
Most fundamental studies of electrocatalysis are based on the experimental and simulation results obtained for bulk model materials. Some of these mechanistic understandings are inapplicable for more active nanostructured electrocatalysts. Herein, considering the simplest and most typical electrocatalytic process, the hydrogen evolution reaction, an alternative reaction mechanism is proposed for nanomaterials based on the identification of a new intermediate, which differs from those commonly known for the bulk counterparts. In-situ Raman spectroscopy and electrochemical thermal/kinetic measurements were conducted on a series of nanomaterials under different conditions. In high-pH electrolytes with negligible hydronium (H3O+) concentration in bulk phase, massive H3O+ intermediates are found generating on the catalytic surface during water dissociation and hydrogen adsorption processes. These H3O+ intermediates create a unique acid-like local reaction environment on nanostructured catalytic surfaces and cut the energy barrier of the overall reaction. Such phenomena on nanostructured electrocatalysts explain their widely observed anomalously high activity under high-pH conditions.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Deng J, Iñiguez JA, Liu C. Electrocatalytic nitrogen reduction at low temperature. Joule. 2018;2:846–856. doi: 10.1016/j.joule.2018.04.014. - DOI
-
- Guo C, Ran J, Vasileff A, Qiao S-Z. Rational design of electrocatalysts and photo (electro) catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci. 2018;11:45–56. doi: 10.1039/C7EE02220D. - DOI
-
- Vasileff A, Xu C, Jiao Y, Zheng Y, Qiao S-Z. Surface and interface engineering in copper-based bimetallic materials for selective CO2 electroreduction. Chem. 2018;4:1809–1831. doi: 10.1016/j.chempr.2018.05.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

