IL-21 regulates SOCS1 expression in autoreactive CD8+ T cells but is not required for acquisition of CTL activity in the islets of non-obese diabetic mice
- PMID: 31653894
- PMCID: PMC6814838
- DOI: 10.1038/s41598-019-51636-5
IL-21 regulates SOCS1 expression in autoreactive CD8+ T cells but is not required for acquisition of CTL activity in the islets of non-obese diabetic mice
Abstract
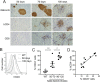
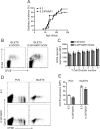
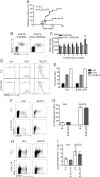
In type 1 diabetes, maturation of activated autoreactive CD8+ T cells to fully armed effector cytotoxic T lymphocytes (CTL) occurs within the islet. At present the signals required for the maturation process are poorly defined. Cytokines could potentially provide the necessary "third signal" required to generate fully mature CTL capable of killing insulin-producing β-cells. To determine whether autoreactive CTL within islets respond to cytokines we generated non-obese diabetic (NOD) mice with a reporter for cytokine signalling. These mice express a reporter gene, hCD4, under the control of the endogenous regulatory elements for suppressor of cytokine signalling (SOCS)1, which is itself regulated by pro-inflammatory cytokines. In NOD mice, the hCD4 reporter was expressed in infiltrated islets and the expression level was positively correlated with the frequency of infiltrating CD45+ cells. SOCS1 reporter expression was induced in transferred β-cell-specific CD8+ 8.3T cells upon migration from pancreatic draining lymph nodes into islets. To determine which cytokines induced SOCS1 promoter activity in islets, we examined hCD4 reporter expression and CTL maturation in the absence of the cytokine receptors IFNAR1 or IL-21R. We show that IFNAR1 deficiency does not confer protection from diabetes in 8.3 TCR transgenic mice, nor is IFNAR1 signalling required for SOCS1 reporter upregulation or CTL maturation in islets. In contrast, IL-21R-deficient 8.3 mice have reduced diabetes incidence and reduced SOCS1 reporter activity in islet CTLs. However IL-21R deficiency did not affect islet CD8+ T cell proliferation or expression of granzyme B or IFNγ. Together these data indicate that autoreactive CD8+ T cells respond to IL-21 and not type I IFNs in the islets of NOD mice, but neither IFNAR1 nor IL-21R are required for islet intrinsic CTL maturation.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

