Impact of Elagolix on Workplace and Household Productivity Among Women with Moderate to Severe Pain Associated with Endometriosis: A Pooled Analysis of Two Phase III Trials
- PMID: 31654294
- PMCID: PMC6884431
- DOI: 10.1007/s40271-019-00394-7
Impact of Elagolix on Workplace and Household Productivity Among Women with Moderate to Severe Pain Associated with Endometriosis: A Pooled Analysis of Two Phase III Trials
Abstract
Background: Endometriosis profoundly impairs women's workplace and household productivity.
Objective: The aim of this study was to evaluate the impact of elagolix on endometriosis-related workplace and household productivity losses.
Methods: Data were pooled from two phase III trials of women aged 18-49 years with moderate to severe endometriosis-associated pain treated for 6 months with elagolix 150 mg daily (QD), 200 mg twice daily (BID), or placebo. The Health-Related Productivity Questionnaire was administered at baseline, Month 3, and Month 6 to determine workplace and household absenteeism and presenteeism. Productivity changes from baseline were compared between placebo and elagolix doses via analysis of covariance.
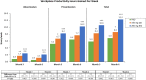
Results: Workplace analyses included 1270 employed women and household analyses included 1565 women. At baseline, women reported average weekly losses of 16 workplace hours, 8.3 household work hours, 45% of scheduled work, and 64% of planned household chores. At Month 6, treatment with elagolix 150 mg QD or 200 mg BID increased productive workplace hours by 1.7 (95% CI 0.1-3.4; p = 0.041) and 5.4 h (95% CI 3.7-7.1; p < 0.001) relative to placebo, corresponding to gains of 5.2% (95% CI 0.7-9.7; p = 0.022) and 14.6% (95% CI 10.0-19.1; p < 0.001) of scheduled work, respectively. Both elagolix doses improved household productivity at Month 6 by 1.7 (95% CI 0.7-2.7) and 3.1 (95% CI 2.1-4.0) hours relative to placebo (both p < 0.001), with increases of 8.8% (95% CI 3.5-14.1; p = 0.001) and 20.4% (95% CI 15.1-25.6; p < 0.001) of planned household work.
Conclusions: Treatment with elagolix improved endometriosis-related workplace and household productivity impairments.
Trial registration: ELARIS EM-I (NCT01620528) and ELARIS EM-II (NCT01931670).
Conflict of interest statement
Eric S. Surrey is Medical Director at Colorado Center for Reproductive Medicine, has served in a consulting role for AbbVie and DOT Laboratories, received research grants from AbbVie, and served on the speaker bureau for AbbVie and Ferring Laboratories. Ahmed M. Soliman is an employee of, owns stock/stock options, and holds patents for AbbVie. Hannah L. Palac was an employee of AbbVie Inc. at the time the research was conducted. Sanjay K. Agarwal is Director of Fertility Services in the UC San Diego Department of Reproductive Medicine, Director of the UC San Diego Center for Endometriosis Research and Treatment, and has served in a consulting role on research for AbbVie Inc. and has received research support from AbbVie Inc.
Figures



References
-
- Abbas S, Ihle P, Koster I, Schubert I. Prevalence and incidence of diagnosed endometriosis and risk of endometriosis in patients with endometriosis-related symptoms: findings from a statutory health insurance-based cohort in Germany. Eur J Obstet Gynecol Reprod Biol. 2012;160(1):79–83. doi: 10.1016/j.ejogrb.2011.09.041. - DOI - PubMed
-
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34(1):41–46. - PubMed

