Mineral-Coated Microparticles Enhance mRNA-Based Transfection of Human Bone Marrow Cells
- PMID: 31655263
- PMCID: PMC6831872
- DOI: 10.1016/j.omtn.2019.09.004
Mineral-Coated Microparticles Enhance mRNA-Based Transfection of Human Bone Marrow Cells
Abstract
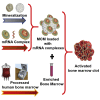
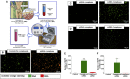
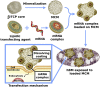
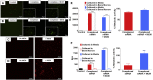
The regenerative potential of bone marrow cells could be harnessed for tissue engineering applications. Bone marrow can be easily collected from patients, providing a valuable autologous source of therapeutic cells. However, years of delivery of bone marrow cells have highlighted the need for their genetic manipulation to overcome heterogeneity and to confer specificity to the regenerative process. In this study, we optimized the use of condensed mRNA as a non-viral alternative. As a proof of concept, we used mRNA encoding for reporter proteins such as EGFP or Firefly luciferase, which was condensed by complexing agents and delivered to human bone marrow cells using mineral-coated microparticles. We demonstrated that human bone marrow cells could be transfected with complexed mRNA, and that this approach was more efficient than the delivery of complexed plasmid DNA. In addition, human bone marrow cells were vulnerable to the toxicity of mRNA complexing agents, but these deleterious effects were mitigated by using mineral-coated microparticles as a carrier of complexed mRNA. Microparticle-mediated delivery of complexed mRNA also enabled higher cell metabolic activity and higher transfection in multiple in vitro culture conditions, including suspension culture and three-dimensional culture.
Keywords: Jurkat; T cells; bone marrow; bone marrow aspirates; gene therapy; mRNA delivery; microparticles; mineral coating; non-viral vectors; transfection.
Copyright © 2019. Published by Elsevier Inc.
Figures


References
-
- Smith B.D., Grande D.A. The current state of scaffolds for musculoskeletal regenerative applications. Nat. Rev. Rheumatol. 2015;11:213–222. - PubMed
-
- Gamie Z., Tran G.T., Vyzas G., Korres N., Heliotis M., Mantalaris A., Tsiridis E. Stem cells combined with bone graft substitutes in skeletal tissue engineering. Expert Opin. Biol. Ther. 2012;12:713–729. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

