Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan
- PMID: 31655492
- PMCID: PMC7003938
- DOI: 10.1111/hepr.13438
Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan
Abstract
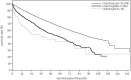
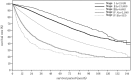
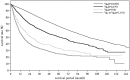
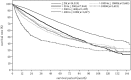
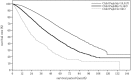
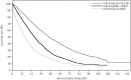
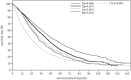
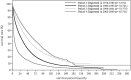
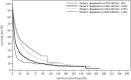
In the 20th Nationwide Follow-up Survey of Primary Liver Cancer in Japan, data from 21 075 new patients and 40 769 previously followed patients were compiled from 544 institutions over a 2-year period from 1 January 2008 to 31 December 2009. Compared with the previous 19th survey, the population of patients with hepatocellular carcinoma (HCC) was older at the time of clinical diagnosis, included more female patients, included more patients with non-B non-C HCC, had smaller tumor diameters and more frequently received radiofrequency ablation as local ablation therapy. Cumulative survival rates were calculated for HCC, intrahepatic cholangiocarcinoma, and combined hepatocellular cholangiocarcinoma (combined HCC and intrahepatic cholangiocarcinoma) by treatment type and by background characteristics for patients newly registered between 1998 and 2009 whose final outcome was survival or death. Cumulative survival rates for HCC were calculated by dividing patients by combinations of background factors (number of tumors, tumor diameter, and Child-Pugh grade) and by treatment types (hepatectomy, local ablation therapy, and transcatheter arterial chemoembolization). Cumulative survival rates and median overall survival in patients treated by resection, transcatheter arterial chemoembolization, and local ablation therapy were calculated. The same values were also calculated by the registration date by dividing patients newly registered between 1978 and 2009 into four time period groups . The results of the analysis show that the prognosis of HCC is improving dramatically. It is expected that the data obtained from this nationwide follow-up survey will contribute to advancing clinical research, including the design of clinical trials, as well as the treatment strategy of primary liver cancer in the clinical practice setting.
Keywords: Liver Cancer Study Group of Japan; combined hepatocellular cholangiocarcinoma; cumulative survival rate; hepatocellular carcinoma; intrahepatic cholangiocarcinoma; nationwide follow-up survey.
© 2019 The Authors. Hepatology Research published by John Wiley & Sons Australia, Ltd on behalf of Japan Society of Hepatology.
Figures

References
-
- Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 1st edn. : Tokyo: Kanehara & Co., Ltd, 1983. (in Japanese).
-
- Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 2nd edn. : Tokyo: Kanehara & Co., Ltd, 1987. (in Japanese).
-
- Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 3rd edn. : Tokyo: Kanehara & Co., Ltd, 1992. (in Japanese).
-
- Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 4th edn. : Tokyo: Kanehara & Co., Ltd, 2000. (in Japanese).
-
- Liver Cancer Study Group of Japan . General rules for the clinical and pathological study of primary liver cancer, 5th edn. : Tokyo: Kanehara & Co., Ltd, 2008. (in Japanese).
LinkOut - more resources
Full Text Sources

