The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors
- PMID: 31655605
- PMCID: PMC6815032
- DOI: 10.1186/s40425-019-0768-9
The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors
Abstract
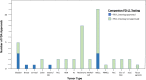
The development of immune checkpoint inhibitors has changed the treatment paradigm for advanced cancers across many tumor types. Despite encouraging and sometimes durable responses in a subset of patients, most patients do not respond. Tumors have adopted the PD-1/PD-L1 axis for immune escape to facilitate tumor growth, which can be leveraged as a potential target for immune checkpoint inhibitors. On this basis, PD-L1 protein expression on tumor or immune cells emerged as the first potential predictive biomarker for sensitivity to immune checkpoint blockade. The goal of our study was to evaluate PD-L1 as a predictive biomarker based on all US Food and Drug Administration (FDA) drug approvals of immune checkpoint inhibitors. We evaluated the primary studies associated with 45 FDA drug approvals from 2011 until April 2019. In total, there were approvals across 15 tumor types. Across all approvals, PD-L1 was predictive in only 28.9% of cases, and was either not predictive (53.3%) or not tested (17.8%) in the remaining cases. There were 9 FDA approvals linked to a specific PD-L1 threshold and companion diagnostic: bladder cancer (N = 3), non-small cell lung cancer (N = 3), triple-negative breast cancer (N = 1), cervical cancer (N = 1), and gastric/gastroesophageal junction cancer (N = 1) with 8 of 9 (88.9%) with immune checkpoint inhibitor monotherapy. The PD-L1 thresholds were variable both within and across tumor types using several different assays, including approvals at the following PD-L1 thresholds: 1, 5, and 50%. PD-L1 expression was also measured in a variable fashion either on tumor cells, tumor-infiltrating immune cells, or both. In conclusion, our findings indicate that PD-L1 expression as a predictive biomarker has limitations and that the decision to pursue testing must be carefully implemented for clinical decision-making.
Keywords: FDA; Immune checkpoint inhibitors; Immunotherapy; PD-L1.
Conflict of interest statement
The authors declare that they have no competing interests relevant to this work.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
