Early treatment with ambrisentan of mildly elevated mean pulmonary arterial pressure associated with systemic sclerosis: a randomized, controlled, double-blind, parallel group study (EDITA study)
- PMID: 31655622
- PMCID: PMC6815440
- DOI: 10.1186/s13075-019-1981-0
Early treatment with ambrisentan of mildly elevated mean pulmonary arterial pressure associated with systemic sclerosis: a randomized, controlled, double-blind, parallel group study (EDITA study)
Abstract
Objective: The objective of this randomized, placebo-controlled, double-blind, parallel group, trial was to assess the effect of ambrisentan on mean pulmonary arterial pressure (mPAP) in patients with systemic sclerosis (SSc) and mildly elevated pulmonary hypertension (PH).
Methods: Thirty-eight SSc patients with mildly elevated mPAP at rest between 21 and 24 mmHg and/or > 30 mmHg during low-dose exercise were randomly assigned to treatment with either ambrisentan 5-10 mg/day or placebo. Right heart catheterization and further clinical parameters were assessed at baseline and after 6 months. The primary endpoint was the difference of mPAP change at rest between groups.
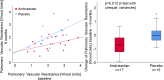
Results: After 6 months, the two groups did not differ in the primary endpoint (ambrisentan mPAP - 1 ± 6.4 mmHg vs. placebo - 0.73 ± 3.59 mmHg at rest, p = 0.884). However, three patients from the placebo group but none of the ambrisentan group progressed to SSc-associated pulmonary arterial hypertension. Furthermore, ambrisentan treatment showed significant improvements in the secondary endpoints cardiac index (CI) and pulmonary vascular resistance (PVR) at rest (CI 0.36 ± 0.66 l/min/m2 vs. - 0.31 ± 0.71 l/min/m2, p = 0.010; PVR - 0.70 ± 0.78 WU vs. 0.01 ± 0.71 WU, p = 0.012) and during exercise (CI 0.7 ± 0.81 l/min/m2 vs. - 0.45 ± 1.36 l/min/m2, p = 0.015; PVR - 0.84 ± 0.48 WU vs. - 0.0032 ± 0.34 WU, p < 0.0001).
Conclusion: This is the first randomized, double-blind, placebo-controlled study testing the effect of ambrisentan in patients with mildly elevated mPAP and/or exercise PH. The primary endpoint change in mPAP did only tendentially improve in the ambrisentan group, but the significant improvement of other hemodynamic parameters points to a possible benefit of ambrisentan and will be helpful to design future trials.
Trial registration: www.ClinicalTrials.gov, unique identifier NCT: NCT02290613 , registered 14th of November 2014.
Keywords: Ambrisentan; Borderline pulmonary hypertension; Exercise PH; Mildly elevated mPAP; Placebo-controlled; Treatment.
Conflict of interest statement
ZP, AMM, CAE, EB, AC, CF, and PX declare that they have no competing interests. NBe has received speaker honoraria from Actelion pharmaceuticals, not related to this study. NoB has received speaker honoraria from Actelion pharmaceuticals, not related to this study. GC reports research support, consultancy fees, and speaker honoraria from Actelion pharmaceuticals. CPD reports speaker honoraria from Actelion pharmaceuticals. OD reports research support and consultancy fees from Actelion pharmaceuticals. BE reports speaker honoraria from Actelion pharmaceuticals. SH reports speaker honoraria from Actelion pharmaceuticals. HML reports speaker and consultancy fees from Actelion pharmaceuticals. EG has received speaker honoraria and advisory board fees from Actelion pharmaceuticals.
Figures



References
-
- Condliffe Robin, Kiely David G., Peacock Andrew J., Corris Paul A., Gibbs J. Simon R., Vrapi Florenc, Das Clare, Elliot Charlie A., Johnson Martin, DeSoyza Julia, Torpy Chantal, Goldsmith Kim, Hodgkins Denise, Hughes Rodney J., Pepke-Zaba Joanna, Coghlan J. Gerry. Connective Tissue Disease–associated Pulmonary Arterial Hypertension in the Modern Treatment Era. American Journal of Respiratory and Critical Care Medicine. 2009;179(2):151–157. doi: 10.1164/rccm.200806-953OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

