Investigating the Osteoinductive Potential of a Decellularized Xenograft Bone Substitute
- PMID: 31655811
- PMCID: PMC6935535
- DOI: 10.1159/000503280
Investigating the Osteoinductive Potential of a Decellularized Xenograft Bone Substitute
Abstract
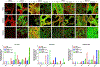
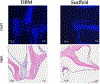
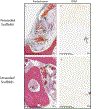
Bone grafting is the second most common tissue transplantation procedure worldwide. One of the alternative methods for bone repair under investigation is a tissue-engineered bone substitute. An ideal property of tissue-engineered bone substitutes is osteoinductivity, defined as the ability to stimulate primitive cells to differentiate into a bone-forming lineage. In the current study, we use a decellularization and oxidation protocol to produce a porcine bone scaffold and examine whether it possesses osteoinductive potential and can be used to create a tissue-engineered bone microenvironment. The decellularization protocol was patented by our lab and consists of chemical decellularization and oxidation steps using combinations of deionized water, trypsin, antimicrobials, peracetic acid, and triton-X100. To test if the bone scaffold was a viable host, preosteoblasts were seeded and analyzed for markers of osteogenic differentiation. The osteoinductive potential was observed in vitro with similar osteogenic markers being expressed in preosteoblasts seeded on the scaffolds and demineralized bone matrix. To assess these properties in vivo, scaffolds with and without preosteoblasts preseeded were subcutaneously implanted in mice for 4 weeks. MicroCT scanning revealed 1.6-fold increased bone volume to total volume ratio and 1.4-fold increase in trabecular thickness in scaffolds after implantation. The histological analysis demonstrates new bone formation and blood vessel formation with pentachrome staining demonstrating osteogenesis and angiogenesis, respectively, within the scaffold. Furthermore, CD31+ staining confirmed the endothelial lining of the blood vessels. These results demonstrate that porcine bone maintains its osteoinductive properties after the application of a patented decellularization and oxidation protocol developed in our laboratory. Future work must be performed to definitively prove osteogenesis of human mesenchymal stem cells, biocompatibility in large animal models, and osteoinduction/osseointegration in a relevant clinical model in vivo. The ability to create a functional bone microenvironment using decellularized xenografts will impact regenerative medicine, orthopedic reconstruction, and could be used in the research of multiple diseases.
Keywords: Angiogenesis; Bone microenvironment; Bone scaffold; Osteoinductivity; Tissue engineering.
© 2019 S. Karger AG, Basel.
Figures





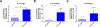


References
-
- Araujo-Gomes N, Romero-Gavilan F, Garcia-Arnaez I, Martinez-Ramos C, Sanchez-Perez AM, Azkargorta M, Elortza F, de Llano JJM, Gurruchaga M, Goni I, Suay J (2018) Osseointegration mechanisms: a proteomic approach. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. - PubMed
-
- Arca T, Proffitt J, Genever P (2011) Generating 3D tissue constructs with mesenchymal stem cells and a cancellous bone graft for orthopaedic applications. Biomed Mater 6(2): 025006. - PubMed
-
- Berner A, Boerckel JD, Saifzadeh S, Steck R, Ren J, Vaquette C, Zhang JQ, Nerlich M, Guldberg RE, Hutmacher DW, Woodruff MA (2012) Biomimetic tubular nanofiber mesh and platelet rich plasma-mediated delivery of BMP-7 for large bone defect regeneration. Cell and tissue research 347(3): 603–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

